Signaling by myeloid C-type lectin receptors in immunity and homeostasis
- PMID: 22224766
- PMCID: PMC4480235
- DOI: 10.1146/annurev-immunol-031210-101352
Signaling by myeloid C-type lectin receptors in immunity and homeostasis
Abstract
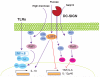
Myeloid cells are key drivers of physiological responses to pathogen invasion or tissue damage. Members of the C-type lectin receptor (CLR) family stand out among the specialized receptors utilized by myeloid cells to orchestrate these responses. CLR ligands include carbohydrate, protein, and lipid components of both pathogens and self, which variably trigger endocytic, phagocytic, proinflammatory, or anti-inflammatory reactions. These varied outcomes rely on a versatile system for CLR signaling that includes tyrosine-based motifs that recruit kinases, phosphatases, or endocytic adaptors as well as nontyrosine-based signals that modulate the activation of other pathways or couple to the uptake machinery. Here, we review the signaling properties of myeloid CLRs and how they impact the role of myeloid cells in innate and adaptive immunity.
Figures



References
-
- Drickamer K. C-type lectin-like domains. Curr Opin Struc Biol. 1999;9:585–90. - PubMed
-
- Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. FEBS J. 2005;272:6179–217. - PubMed
-
- Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. - PubMed
-
- Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol. 2006;7:1258–65. - PubMed
-
- Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity. 2011;34:651–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

