The response to and repair of RAG-mediated DNA double-strand breaks
- PMID: 22224778
- PMCID: PMC4038028
- DOI: 10.1146/annurev-immunol-030409-101320
The response to and repair of RAG-mediated DNA double-strand breaks
Abstract
Developing lymphocytes must assemble antigen receptor genes encoding the B cell and T cell receptors. This process is executed by the V(D)J recombination reaction, which can be divided into DNA cleavage and DNA joining steps. The former is carried out by a lymphocyte-specific RAG endonuclease, which mediates DNA cleavage at two recombining gene segments and their flanking RAG recognition sequences. RAG cleavage generates four broken DNA ends that are repaired by nonhomologous end joining forming coding and signal joints. On rare occasions, these DNA ends may join aberrantly forming chromosomal lesions such as translocations, deletions and inversions that have the potential to cause cellular transformation and lymphoid tumors. We discuss the activation of DNA damage responses by RAG-induced DSBs focusing on the component pathways that promote their normal repair and guard against their aberrant resolution. Moreover, we discuss how this DNA damage response impacts processes important for lymphocyte development.
Figures

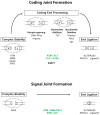

References
-
- Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG. The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu Rev Immunol. 2000;18:495–527. - PubMed
-
- Oettinger MA. V(D)J recombination: on the cutting edge. Curr Opin Cell Biol. 1999;11:325–9. - PubMed
-
- Gellert M. V(D)J recombination: rag proteins, repair factors, and regulation. Annu Rev Biochem. 2002;71:101–32. - PubMed
-
- Desiderio S, Lin WC, Li Z. The cell cycle and V(D)J recombination. Curr Top Microbiol Immunol. 1996;217:45–59. - PubMed
-
- Rooney S, Chaudhuri J, Alt FW. The role of the non-homologous end-joining pathway in lymphocyte development. Immunol Rev. 2004;200:115–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

