Synthesis of thiophenylalanine-containing peptides via Cu(I)-mediated cross-coupling
- PMID: 22224916
- PMCID: PMC3725732
- DOI: 10.1021/ol202947f
Synthesis of thiophenylalanine-containing peptides via Cu(I)-mediated cross-coupling
Abstract
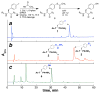
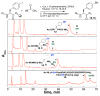
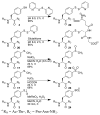
Aryl thiolates have unique reactive, redox, electronic, and spectroscopic properties. A practical approach to synthesize peptides containing thiophenylalanine has been developed via a novel Cu(I)-mediated cross-coupling reaction between thiolacetic acid and iodophenylalanine-containing peptides in the solid phase. This approach is compatible with all canonical proteinogenic functional groups, providing general access to aryl thiolates in peptides. Peptides containing thiophenylalanine (pK(a) 6.4) were readily elaborated to contain methyl, allyl, and nitrobenzyl thioethers, disulfides, sulfoxides, sulfones, or sulfonates.
© 2012 American Chemical Society
Figures



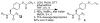
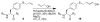
Similar articles
-
Ligand-free copper-catalyzed C-S coupling of aryl iodides and thiols.J Org Chem. 2008 Jul 18;73(14):5625-8. doi: 10.1021/jo800491k. Epub 2008 Jun 21. J Org Chem. 2008. PMID: 18570479
-
A general and efficient approach to aryl thiols: CuI-catalyzed coupling of aryl iodides with sulfur and subsequent reduction.Org Lett. 2009 Nov 19;11(22):5250-3. doi: 10.1021/ol902186d. Org Lett. 2009. PMID: 19835369
-
Synthesis of CuO on mesoporous silica and its applications for coupling reactions of thiols with aryl iodides.Chem Commun (Camb). 2010 Jan 14;46(2):282-4. doi: 10.1039/b918117b. Epub 2009 Nov 25. Chem Commun (Camb). 2010. PMID: 20024352
-
The Cysteine S-Alkylation Reaction as a Synthetic Method to Covalently Modify Peptide Sequences.Chemistry. 2017 Jan 5;23(2):224-233. doi: 10.1002/chem.201602694. Epub 2016 Aug 19. Chemistry. 2017. PMID: 27538566 Review.
-
Synthesis of sulfonopeptides.J Pept Sci. 2021 Aug;27(8):e3331. doi: 10.1002/psc.3331. Epub 2021 Apr 28. J Pept Sci. 2021. PMID: 33913204 Review.
Cited by
-
Proline editing: a general and practical approach to the synthesis of functionally and structurally diverse peptides. Analysis of steric versus stereoelectronic effects of 4-substituted prolines on conformation within peptides.J Am Chem Soc. 2013 Mar 20;135(11):4333-63. doi: 10.1021/ja3109664. Epub 2013 Mar 11. J Am Chem Soc. 2013. PMID: 23402492 Free PMC article.
-
4R- and 4S-iodophenyl hydroxyproline, 4R-pentynoyl hydroxyproline, and S-propargyl-4-thiolphenylalanine: conformationally biased and tunable amino acids for bioorthogonal reactions.Org Biomol Chem. 2016 Feb 21;14(7):2327-46. doi: 10.1039/c5ob02473k. Epub 2016 Jan 25. Org Biomol Chem. 2016. PMID: 26806113 Free PMC article.
-
Aromatic-proline interactions: electronically tunable CH/π interactions.Acc Chem Res. 2013 Apr 16;46(4):1039-49. doi: 10.1021/ar300087y. Epub 2012 Nov 13. Acc Chem Res. 2013. PMID: 23148796 Free PMC article.
-
Synthesis of Perfluoro-tert-butyl Tyrosine, for Application in 19F NMR, via a Diazonium-Coupling Reaction.Org Lett. 2016 Dec 16;18(24):6240-6243. doi: 10.1021/acs.orglett.6b02858. Epub 2016 Dec 6. Org Lett. 2016. PMID: 27978684 Free PMC article.
-
Streamlined construction of peptide macrocycles via palladium-catalyzed intramolecular S-arylation in solution and on DNA.Chem Sci. 2021 Mar 11;12(16):5804-5810. doi: 10.1039/d1sc00789k. Chem Sci. 2021. PMID: 34168804 Free PMC article.
References
-
- Leonard SE, Carroll KS. Curr Opin Chem Biol. 2011;15:88–102. - PubMed
- Paulsen CE, Carroll KS. ACS Chem Biol. 2010;5:47–62. - PMC - PubMed
- Reddie KG, Carroll KS. Curr Opin Chem Biol. 2008;12:746–54. - PubMed
- Leonard SE, Garcia FJ, Goodsell DS, Carroll KS. Angew Chem Int Ed. 2011;50:4423–7. - PubMed
- Cline DJ, Thorpe C, Schneider JP. Anal Biochem. 2004;325:144–50. - PubMed
- Kodali VK, Thorpe C. Antioxid Redox Signaling. 2010;13:1217–30. - PMC - PubMed
- Berg JM, Godwin HA. Ann Rev Biophys Biomol Struct. 1997;26:357–71. - PubMed
- Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, Llopis J, Tsien RY. J Am Chem Soc. 2002;124:6063–76. - PubMed
- Chalker JM, Bernardes GJL, Lin YA, Davis BG. Chem Asian J. 2009;4:630–40. - PubMed
- Dawson PE, Muir TW, Clark-Lewis I, Kent SBH. Science. 1994;266:776–9. - PubMed
-
-
Application of aryl thiolates to enhance protein folding rates at pH 6: Gough JD, Williams JRH, Donofrio AE, Lees WJ. J Am Chem Soc. 2002;124:3885–92.
-
-
- Escher E, Bernier M, Parent P. Helv Chim Acta. 1983;66:1355–65.
- Guillemette G, Bernier M, Parent P, Leduc R, Escher E. J Med Chem. 1984;27:315–20. - PubMed
-
Other syntheses of 4-ThioPhe: Johnson TB, Brautlecht CA. J Biol Chem. 1912;12:175–96.
- Elliott DF, Harrington C. J Chem Soc. 1949:1374–8.
- Colescott RL, Herr RR, Dailey JP. J Am Chem Soc. 1957;79:4232–5.
- Bergel F, Stock JA. J Chem Soc. 1959:90–7.
-
Synthesis of S-t-butyl Boc-ThioPhe via Pd cross-coupling: Rajagopalan S, Radke G, Evans M, Tomich JM. Synth Commun. 1996;26:1431–40.
-
- Lu HSM, Volk M, Kholodenko Y, Gooding E, Hochstrasser RM, DeGrado WF. J Am Chem Soc. 1997;119:7173–80.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

