3-Iodothyronamine: a modulator of the hypothalamus-pancreas-thyroid axes in mice
- PMID: 22225569
- PMCID: PMC3417495
- DOI: 10.1111/j.1476-5381.2011.01823.x
3-Iodothyronamine: a modulator of the hypothalamus-pancreas-thyroid axes in mice
Abstract
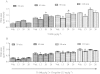
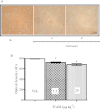
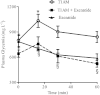
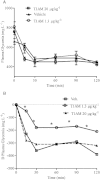
BACKGROUND AND PURPOSE Preclinical pharmacology of 3-iodothyronamine (T1AM), an endogenous derivative of thyroid hormones, indicates that it is a rapid modulator of rodent metabolism and behaviour. Since T1AM undergoes rapid enzymatic degradation, particularly by MAO, we hypothesized that the effects of T1AM might be altered by inhibition of MAO. EXPERIMENTAL APPROACH We investigated the effects of injecting T1AM (i.c.v.) on (i) feeding behaviour, hyperglycaemia and plasma levels of thyroid hormones and (ii) T1AM systemic bioavailability, in overnight fasted mice, under control conditions and after pretreatment with the MAO inhibitor clorgyline. T1AM (1.3, 6.6, 13, 20 and 26 µg·kg(-1) ) or vehicle were injected i.c.v. in fasted male mice not pretreated or pretreated i.p. with clorgyline (2.5 mg·kg(-1) ). Glycaemia was measured by a glucorefractometer, plasma triiodothyronine (fT3) by a chemiluminescent immunometric assay, c-fos activation immunohistochemically and plasma T1AM by HPLC coupled to tandem-MS. KEY RESULTS T1AM, 1.3 µg·kg(-1) , produced a hypophagic effect (-24% vs. control) and reduced c-fos activation. This dose showed systemic bioavailability (0.12% of injected dose), raised plasma glucose levels and reduced peripheral insulin sensitivity (-33% vs. control) and plasma fT3 levels. These effects were not linearly related to the dose injected. Clorgyline pretreatment strongly increased the systemic bioavailability of T1AM and prevented the hyperglycaemia and reduction in fT3 induced by T1AM. CONCLUSIONS AND IMPLICATIONS T1AM induces central and peripheral effects including hyperglycaemia and a reduction in plasma fT3 levels in fasted mice. These effects critically depend on the concentration of T1AM or its metabolites in target organs.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures

References
-
- Becskei C, Lutz TA, Riediger T. Reduced fasting-induced activation of hypothalamic arcuate neurons is associated with hyperleptinemia and increased leptin sensitivity in obese mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R632–R641. - PubMed
-
- Dhillo WS, Bewick GA, White NE, Gardiner JV, Thompson EL, Bataveljic A, et al. The thyroid hormone derivative 3-iodothyronamine increases food intake in rodents. Diabetes Obes Metab. 2009;11:251–260. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

