Central coordination as an alternative for local coordination in a multicenter randomized controlled trial: the FAITH trial experience
- PMID: 22225733
- PMCID: PMC3275506
- DOI: 10.1186/1745-6215-13-5
Central coordination as an alternative for local coordination in a multicenter randomized controlled trial: the FAITH trial experience
Abstract
Background: Surgeons in the Netherlands, Canada and the US participate in the FAITH trial (Fixation using Alternative Implants for the Treatment of Hip fractures). Dutch sites are managed and visited by a financed central trial coordinator, whereas most Canadian and US sites have local study coordinators and receive per patient payment. This study was aimed to assess how these different trial management strategies affected trial performance.
Methods: Details related to obtaining ethics approval, time to trial start-up, inclusion, and percentage completed follow-ups were collected for each trial site and compared. Pre-trial screening data were compared with actual inclusion rates.
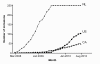
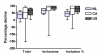
Results: Median trial start-up ranged from 41 days (P25-P75 10-139) in the Netherlands to 232 days (P25-P75 98-423) in Canada (p = 0.027). The inclusion rate was highest in the Netherlands; median 1.03 patients (P25-P75 0.43-2.21) per site per month, representing 34.4% of the total eligible population. It was lowest in Canada; 0.14 inclusions (P25-P75 0.00-0.28), representing 3.9% of eligible patients (p < 0.001). The percentage completed follow-ups was 83% for Canadian and Dutch sites and 70% for US sites (p = 0.217).
Conclusions: In this trial, a central financed trial coordinator to manage all trial related tasks in participating sites resulted in better trial progression and a similar follow-up. It is therefore a suitable alternative for appointing these tasks to local research assistants. The central coordinator approach can enable smaller regional hospitals to participate in multicenter randomized controlled trials. Circumstances such as available budget, sample size, and geographical area should however be taken into account when choosing a management strategy.
Trial registration: ClinicalTrials.gov: NCT00761813.
Figures
References
-
- Bhandari M, Schemitsch EH. Beyond the basics: the organization and coordination of multicenter trials. Tech Orthop. 2004;19:83–87. doi: 10.1097/00013611-200406000-00007. - DOI
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

