Protein translocation through Tom40: kinetics of peptide release
- PMID: 22225796
- PMCID: PMC3250685
- DOI: 10.1016/j.bpj.2011.11.4003
Protein translocation through Tom40: kinetics of peptide release
Abstract
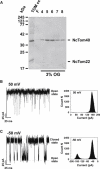
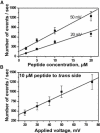
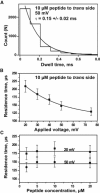
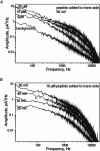
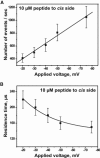
Mitochondrial proteins are almost exclusively imported into mitochondria from the cytosol in an unfolded or partially folded conformation. Regardless of whether they are destined for the outer or inner membrane, the intermembrane space, or the matrix, proteins begin the importation process by crossing the mitochondrial outer membrane via a specialized protein import machinery whose main component is the Tom40 channel. High-resolution ion conductance measurements through the Tom40 channel in the presence of the mitochondrial presequence peptide pF(1)β revealed the kinetics of peptide binding. Here we show that the rates for association k(on) and dissociation k(off) strongly depend on the applied transmembrane voltage. Both kinetic constants increase with an increase in the applied voltage. The increase of k(off) with voltage provides strong evidence of peptide translocation. This allows us to distinguish quantitatively between substrate blocking and permeation.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Pfanner N., Geissler A. Versatility of the mitochondrial protein import machinery. Nat. Rev. Mol. Cell Biol. 2001;2:339–349. - PubMed
-
- Neupert W., Herrmann J.M. Translocation of proteins into mitochondria. Annu. Rev. Biochem. 2007;76:723–749. - PubMed
-
- Mokranjac D., Neupert W. Thirty years of protein translocation into mitochondria: unexpectedly complex and still puzzling. Biochim. Biophys. Acta. 2009;1793:33–41. - PubMed
-
- Schleiff E., Becker T. Common ground for protein translocation: access control for mitochondria and chloroplasts. Nat. Rev. Mol. Cell Biol. 2011;12:48–59. - PubMed
-
- Endo T., Yamano K., Kawano S. Structural insight into the mitochondrial protein import system. Biochim. Biophys. Acta. 2011;1808:955–970. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

