α-α Cross-links increase fibrin fiber elasticity and stiffness
- PMID: 22225811
- PMCID: PMC3250683
- DOI: 10.1016/j.bpj.2011.11.4016
α-α Cross-links increase fibrin fiber elasticity and stiffness
Abstract
Fibrin fibers, which are ~100 nm in diameter, are the major structural component of a blood clot. The mechanical properties of single fibrin fibers determine the behavior of a blood clot and, thus, have a critical influence on heart attacks, strokes, and embolisms. Cross-linking is thought to fortify blood clots; though, the role of α-α cross-links in fibrin fiber assembly and their effect on the mechanical properties of single fibrin fibers are poorly understood. To address this knowledge gap, we used a combined fluorescence and atomic force microscope technique to determine the stiffness (modulus), extensibility, and elasticity of individual, uncross-linked, exclusively α-α cross-linked (γQ398N/Q399N/K406R fibrinogen variant), and completely cross-linked fibrin fibers. Exclusive α-α cross-linking results in 2.5× stiffer and 1.5× more elastic fibers, whereas full cross-linking results in 3.75× stiffer, 1.2× more elastic, but 1.2× less extensible fibers, as compared to uncross-linked fibers. On the basis of these results and data from the literature, we propose a model in which the α-C region plays a significant role in inter- and intralinking of fibrin molecules and protofibrils, endowing fibrin fibers with increased stiffness and elasticity.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

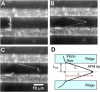
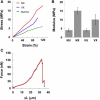
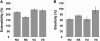
Similar articles
-
Fibrin mechanical properties and their structural origins.Matrix Biol. 2017 Jul;60-61:110-123. doi: 10.1016/j.matbio.2016.08.003. Epub 2016 Aug 20. Matrix Biol. 2017. PMID: 27553509 Free PMC article. Review.
-
Fibrin Fiber Stiffness Is Strongly Affected by Fiber Diameter, but Not by Fibrinogen Glycation.Biophys J. 2016 Mar 29;110(6):1400-10. doi: 10.1016/j.bpj.2016.02.021. Biophys J. 2016. PMID: 27028649 Free PMC article.
-
Factor XIII stiffens fibrin clots by causing fiber compaction.J Thromb Haemost. 2014 Oct;12(10):1687-96. doi: 10.1111/jth.12705. Epub 2014 Sep 18. J Thromb Haemost. 2014. PMID: 25142383
-
Recombinant fibrinogen reveals the differential roles of α- and γ-chain cross-linking and molecular heterogeneity in fibrin clot strain-stiffening.J Thromb Haemost. 2017 May;15(5):938-949. doi: 10.1111/jth.13650. Epub 2017 Mar 6. J Thromb Haemost. 2017. PMID: 28166607
-
A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers.Cell Biochem Biophys. 2007;49(3):165-81. doi: 10.1007/s12013-007-9001-4. Epub 2007 Oct 2. Cell Biochem Biophys. 2007. PMID: 17952642 Free PMC article. Review.
Cited by
-
Fibrin mechanical properties and their structural origins.Matrix Biol. 2017 Jul;60-61:110-123. doi: 10.1016/j.matbio.2016.08.003. Epub 2016 Aug 20. Matrix Biol. 2017. PMID: 27553509 Free PMC article. Review.
-
Newly-Recognized Roles of Factor XIII in Thrombosis.Semin Thromb Hemost. 2016 Jun;42(4):445-54. doi: 10.1055/s-0036-1571343. Epub 2016 Apr 7. Semin Thromb Hemost. 2016. PMID: 27056150 Free PMC article. Review.
-
Transglutaminase Activities of Blood Coagulant Factor XIII Are Dependent on the Activation Pathways and on the Substrates.Thromb Haemost. 2023 Apr;123(4):380-392. doi: 10.1055/a-1993-4193. Epub 2022 Dec 6. Thromb Haemost. 2023. PMID: 36473493 Free PMC article.
-
Nonuniform Internal Structure of Fibrin Fibers: Protein Density and Bond Density Strongly Decrease with Increasing Diameter.Biomed Res Int. 2017;2017:6385628. doi: 10.1155/2017/6385628. Epub 2017 Oct 10. Biomed Res Int. 2017. PMID: 29130043 Free PMC article.
-
Fibrin Fiber Stiffness Is Strongly Affected by Fiber Diameter, but Not by Fibrinogen Glycation.Biophys J. 2016 Mar 29;110(6):1400-10. doi: 10.1016/j.bpj.2016.02.021. Biophys J. 2016. PMID: 27028649 Free PMC article.
References
-
- Lorand L. Factor XIII: structure, activation, and interactions with fibrinogen and fibrin. Ann. N. Y. Acad. Sci. 2001;936:291–311. - PubMed
-
- Deguchi S., Ohashi T., Sato M. Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. J. Biomech. 2006;39:2603–2610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

