Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials
- PMID: 22225814
- PMCID: PMC3420069
- DOI: 10.1016/S0140-6736(11)61750-9
Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials
Erratum in
- Lancet. 2012 Feb 18;379(9816):616
Abstract
Background: Skin and mucosal herpes simplex virus type 2 (HSV-2) shedding predominantly occurs in short subclinical episodes. We assessed whether standard-dose or high-dose antiviral therapy reduces the frequency of such shedding.
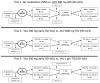
Methods: HSV-2-seropositive, HIV-seronegative people were enrolled at the University of Washington Virology Research Clinic (WA, USA). We did three separate but complementary open-label cross-over studies comparing no medication with aciclovir 400 mg twice daily (standard-dose aciclovir), valaciclovir 500 mg daily (standard-dose valaciclovir) with aciclovir 800 mg three times daily (high-dose aciclovir), and standard-dose valaciclovir with valaciclovir 1 g three times daily (high-dose valaciclovir). The allocation sequence was generated by a random number generator. Study drugs were supplied in identical, numbered, sealed boxes. Study periods lasted 4-7 weeks, separated by 1 week wash-out. Participants collected genital swabs four times daily for quantitative HSV DNA PCR. Clinical data were masked from laboratory personnel. The primary endpoint was within-person comparison of shedding rate in each study group. Analysis was per protocol. The trials are registered at ClinicalTrials.gov (NCT00362297, NCT00723229, NCT01346475).
Results: Of 113 participants randomised, 90 were eligible for analysis of the primary endpoint. Participants collected 23 605 swabs; 1272 (5·4%) were HSV-positive. The frequency of HSV shedding was significantly higher in the no medication group (n=384, 18·1% of swabs) than in the standard-dose aciclovir group (25, 1·2%; incidence rate ratio [IRR] 0·05, 95% CI 0·03-0·08). High-dose aciclovir was associated with less shedding than standard-dose valaciclovir (198 [4·2%] vs 209 [4·5%]; IRR 0·79, 95% CI 0·63-1·00). Shedding was less frequent in the high-dose valaciclovir group than in the standard-dose valaciclovir group (164 [3·3%] vs 292 [5·8%]; 0·54, 0·44-0·66). The number of episodes per person-year did not differ significantly for standard-dose valaciclovir (22·6) versus high-dose aciclovir (20·2; p=0·54), and standard-dose valaciclovir (14·9) versus high-dose valaciclovir (16·5; p=0·34), but did for no medication (28·7) and standard-dose aciclovir (10·0; p=0·001). Median episode duration was longer for no medication than for standard-dose aciclovir (13 h vs 7 h; p=0·01) and for standard-dose valaciclovir than for high-dose valaciclovir (10 h vs 7 h; p=0·03), but did not differ significantly between standard-dose valaciclovir and high-dose aciclovir (8 h vs 8 h; p=0·23). Likewise, maximum log(10) copies of HSV detected per mL was higher for no medication than for standard dose aciclovir (3·3 vs 2·9; p=0·02), and for standard-dose valaciclovir than for high-dose valaciclovir (2·5 vs 3·0; p=0·001), but no significant difference was recorded for standard-dose valaciclovir versus high-dose aciclovir (2·7 vs 2·8; p=0·66). 80% of episodes were subclinical in all study groups. Except for a higher frequency of headaches with high-dose valaciclovir (n=13, 30%) than with other regimens, all regimens were well tolerated.
Interpretation: Short bursts of subclinical genital HSV reactivation are frequent, even during high-dose antiherpes therapy, and probably account for continued transmission of HSV during suppressive antiviral therapy. More potent antiviral therapy is needed to eliminate HSV transmission.
Funding: NIH. Valaciclovir was provided for trial 3 for free by GlaxoSmithKline.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CJ is a research investigator for AiCuris, GmbH. AW and LC are consultants for AiCuris GmbH, which is developing treatments for HSV. LC is the head of the scientific advisory board for and holds stock (<1% of company) in Immune Design Corp. LC and DMK are listed as coinventors on several patents describing antigens and epitopes to which T-cell responses to HSV-2 are directed.
AM and DMK are consultants for Immune Design Corp.
DMK is a consultant to Sanofi-Pasteur and Agenus Inc, for HSV-2 vaccines, and is contracted by Coridon Pty Ltd, Vical, Inc, and PATH for preclinical evaluation of a candidate HSV-2 vaccine. MS, SK, SS, MLH, JTS no conflicts.
Figures
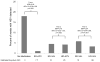
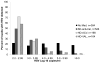
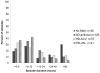
Comment in
-
Herpes simplex virus: a new era?Lancet. 2012 Feb 18;379(9816):598-9. doi: 10.1016/S0140-6736(11)61614-0. Epub 2012 Jan 4. Lancet. 2012. PMID: 22226046 No abstract available.
-
Infection: high-dose antiviral therapy does not prevent short episodes of HSV-2 reactivation.Nat Rev Urol. 2012 Mar 13;9(4):178. doi: 10.1038/nrurol.2012.35. Nat Rev Urol. 2012. PMID: 22410677 No abstract available.
Similar articles
-
Valaciclovir versus aciclovir in patient initiated treatment of recurrent genital herpes: a randomised, double blind clinical trial. International Valaciclovir HSV Study Group.Genitourin Med. 1997 Apr;73(2):110-6. doi: 10.1136/sti.73.2.110. Genitourin Med. 1997. PMID: 9215092 Free PMC article. Clinical Trial.
-
Valaciclovir: a review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections.Drugs. 2000 Apr;59(4):839-63. doi: 10.2165/00003495-200059040-00013. Drugs. 2000. PMID: 10804039 Review.
-
Effect of Pritelivir Compared With Valacyclovir on Genital HSV-2 Shedding in Patients With Frequent Recurrences: A Randomized Clinical Trial.JAMA. 2016 Dec 20;316(23):2495-2503. doi: 10.1001/jama.2016.18189. JAMA. 2016. PMID: 27997653 Clinical Trial.
-
High-dose valacyclovir decreases plasma HIV-1 RNA more than standard-dose acyclovir in persons coinfected with HIV-1 and HSV-2: a randomized crossover trial.J Acquir Immune Defic Syndr. 2013 Jun 1;63(2):201-8. doi: 10.1097/QAI.0b013e3182928eea. J Acquir Immune Defic Syndr. 2013. PMID: 23542637 Free PMC article. Clinical Trial.
-
Valaciclovir. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections.Drugs. 1996 Nov;52(5):754-72. doi: 10.2165/00003495-199652050-00009. Drugs. 1996. PMID: 9118821 Review.
Cited by
-
Immunoregulatory Functions of Interferons During Genital HSV-2 Infection.Front Immunol. 2021 Aug 18;12:724618. doi: 10.3389/fimmu.2021.724618. eCollection 2021. Front Immunol. 2021. PMID: 34484233 Free PMC article. Review.
-
Biologic interactions between HSV-2 and HIV-1 and possible implications for HSV vaccine development.Vaccine. 2019 Nov 28;37(50):7363-7371. doi: 10.1016/j.vaccine.2017.09.044. Epub 2017 Sep 25. Vaccine. 2019. PMID: 28958807 Free PMC article. Review.
-
Global and regional estimates of the contribution of herpes simplex virus type 2 infection to HIV incidence: a population attributable fraction analysis using published epidemiological data.Lancet Infect Dis. 2020 Feb;20(2):240-249. doi: 10.1016/S1473-3099(19)30470-0. Epub 2019 Nov 18. Lancet Infect Dis. 2020. PMID: 31753763 Free PMC article.
-
Infection: high-dose antiviral therapy does not prevent short episodes of HSV-2 reactivation.Nat Rev Urol. 2012 Mar 13;9(4):178. doi: 10.1038/nrurol.2012.35. Nat Rev Urol. 2012. PMID: 22410677 No abstract available.
-
Enhancement of herpes simplex virus (HSV) infection by seminal plasma and semen amyloids implicates a new target for the prevention of HSV infection.Viruses. 2015 Apr 20;7(4):2057-73. doi: 10.3390/v7042057. Viruses. 2015. PMID: 25903833 Free PMC article.
References
-
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73– 83. - PubMed
-
- Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. Jama. 2006;296(8):964–73. - PubMed
-
- Morris SR, Bauer HM, Samuel MC, Gallagher D, Bolan G. Neonatal Herpes Morbidity and Mortality in California, 1995–2003. Sexually Transmitted Diseases. 2008;35(1):14–8. 0.1097/OLQ.0b013e3180f62bc7. - PubMed
-
- Mertz GJ, Jones CC, Mills J, et al. Long-term acyclovir suppression of frequently recurring genital herpes simplex virus infection. A multicenter double-blind trial. Jama. 1988;260(2):201–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

