Comparison of the effects of estradiol and progesterone on serotonergic function
- PMID: 22225849
- PMCID: PMC3307822
- DOI: 10.1016/j.biopsych.2011.11.023
Comparison of the effects of estradiol and progesterone on serotonergic function
Abstract
Background: Ovarian hormones may contribute to the vulnerability to depression, as well as to the response to antidepressants (ADs). Previously, we reported that acute systemic treatment with estradiol or progesterone blocked the ability of the selective serotonin reuptake inhibitor, fluvoxamine, to inhibit serotonin transporter function in ovariectomized rats. In this study, behavioral consequences, as well as receptor mechanisms underlying these hormonal effects, were investigated.
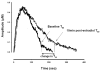
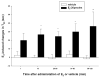
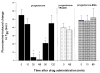
Methods: Using the forced swimming test, the acute effect of estradiol and/or progesterone on fluvoxamine's AD-like effects was investigated. Using in vivo chronoamperometry, the effect of local application of estradiol or progesterone into the hippocampus of ovariectomized rats on serotonin (5-HT) clearance, as well as on the ability of fluvoxamine to slow 5-HT clearance, were investigated.
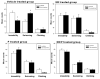
Results: The decreased immobility and increased swimming caused by fluvoxamine in the forced swimming test was blocked in rats treated with estradiol and/or progesterone. Local application of estradiol, but not progesterone, slowed 5-HT clearance and both hormones blocked the ability of fluvoxamine to slow 5-HT clearance. Use of hormone receptor agonists and antagonists, revealed that the effects of estradiol are mediated by activation of membrane, as well as nuclear estrogen receptors (ER). The AD-like effect of estradiol involved ER beta and G-protein coupled receptor 30, whereas its blockade of fluvoxamine's effects was ER alpha-mediated. The effects of progesterone occurred solely by activation of intracellular progesterone receptors.
Conclusions: Targeting of ER beta or G-protein coupled receptor 30 might reveal a strategy to permit beneficial effects of estrogen without its deleterious effect on selective serotonin reuptake inhibitor efficacy.
Copyright © 2012 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
FINANCIAL DISCLOSURES: Dr. Benmansour, Ms. Weaver, Ms. Barton and Mr. Adeniji have no biomedical financial interests or potential conflicts of interest.
Dr. Frazer has been on advisory boards for Cyberonics, Inc., H. Lundbeck A/S and Takeda Pharmaceuticals America, Inc. and he has consulted and/or received research support for preclinical studies from Forest Research Institute, Eli Lilly and Company, Wyeth Pharmaceuticals, and H. Lundbeck A/S. No support for this study was received from any pharmaceutical company.
Figures
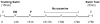
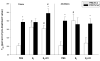
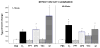

Similar articles
-
Impact of ovarian hormones on the modulation of the serotonin transporter by fluvoxamine.Neuropsychopharmacology. 2009 Feb;34(3):555-64. doi: 10.1038/npp.2008.23. Epub 2008 Mar 5. Neuropsychopharmacology. 2009. PMID: 18322468
-
Signaling mechanisms involved in the acute effects of estradiol on 5-HT clearance.Int J Neuropsychopharmacol. 2014 May;17(5):765-77. doi: 10.1017/S146114571300165X. Epub 2014 Jan 15. Int J Neuropsychopharmacol. 2014. PMID: 24423185 Free PMC article.
-
Effects of Long-Term Treatment with Estradiol and Estrogen Receptor Subtype Agonists on Serotonergic Function in Ovariectomized Rats.Neuroendocrinology. 2016;103(3-4):269-81. doi: 10.1159/000437268. Epub 2015 Jul 1. Neuroendocrinology. 2016. PMID: 26159182 Free PMC article.
-
Serotonin transporter function in vivo: assessment by chronoamperometry.Ann N Y Acad Sci. 1998 Dec 15;861:217-29. doi: 10.1111/j.1749-6632.1998.tb10193.x. Ann N Y Acad Sci. 1998. PMID: 9928259 Review.
-
Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice.Neuropharmacology. 2008 Nov;55(6):1006-14. doi: 10.1016/j.neuropharm.2008.08.001. Epub 2008 Aug 12. Neuropharmacology. 2008. PMID: 18761360 Review.
Cited by
-
The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle.J Abnorm Psychol. 2013 Feb;122(1):131-7. doi: 10.1037/a0029524. Epub 2012 Aug 13. J Abnorm Psychol. 2013. PMID: 22889242 Free PMC article.
-
Serum levels of GPER-1 in euthymic bipolar patients.Neuropsychiatr Dis Treat. 2018 Mar 26;14:855-862. doi: 10.2147/NDT.S158822. eCollection 2018. Neuropsychiatr Dis Treat. 2018. PMID: 29618927 Free PMC article.
-
Greater monoamine oxidase a binding in perimenopausal age as measured with carbon 11-labeled harmine positron emission tomography.JAMA Psychiatry. 2014 Aug;71(8):873-9. doi: 10.1001/jamapsychiatry.2014.250. JAMA Psychiatry. 2014. PMID: 24898155 Free PMC article.
-
Oral contraceptives and the serotonin 4 receptor: a molecular brain imaging study in healthy women.Acta Psychiatr Scand. 2020 Oct;142(4):294-306. doi: 10.1111/acps.13211. Epub 2020 Jul 21. Acta Psychiatr Scand. 2020. PMID: 33314049 Free PMC article.
-
The pathophysiology of estrogen in perinatal depression: conceptual update.Arch Womens Ment Health. 2024 Dec;27(6):887-897. doi: 10.1007/s00737-024-01494-6. Epub 2024 Aug 3. Arch Womens Ment Health. 2024. PMID: 39096394 Review.
References
-
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev. 2008;115:291–313. - PubMed
-
- Pearlstein TB. Hormones and depression: what are the facts about premenstrual syndrome, menopause, and hormone replacement therapy? Am J Obstet Gynecol. 1995;173:646–653. - PubMed
-
- Amore M, Di Donato P, Berti A, Palareti A, Chirico C, Papalini A, et al. Sexual and psychological symptoms in the climacteric years. Maturitas. 2007;56:303–311. - PubMed
-
- Soares CN. Depression during the menopausal transition: window of vulnerability or continuum of risk? Menopause. 2008;15:207–209. - PubMed
-
- Frey BN, Lord C, Soares CN. Depression during menopausal transition: a review of treatment strategies and pathophysiological correlates. Menopause Int. 2008;14:123–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

