Neurogenesis in the central olfactory pathway of adult decapod crustaceans: development of the neurogenic niche in the brains of procambarid crayfish
- PMID: 22225949
- PMCID: PMC3266201
- DOI: 10.1186/1749-8104-7-1
Neurogenesis in the central olfactory pathway of adult decapod crustaceans: development of the neurogenic niche in the brains of procambarid crayfish
Abstract
Background: In the decapod crustacean brain, neurogenesis persists throughout the animal's life. After embryogenesis, the central olfactory pathway integrates newborn olfactory local and projection interneurons that replace old neurons or expand the existing population. In crayfish, these neurons are the descendants of precursor cells residing in a neurogenic niche. In this paper, the development of the niche was documented by monitoring proliferating cells with S-phase-specific markers combined with immunohistochemical, dye-injection and pulse-chase experiments.
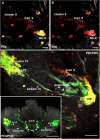
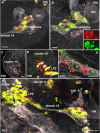
Results: Between the end of embryogenesis and throughout the first post-embryonic stage (POI), a defined transverse band of mitotically active cells (which we will term 'the deutocerebral proliferative system' (DPS) appears. Just prior to hatching and in parallel with the formation of the DPS, the anlagen of the niche appears, closely associated with the vasculature. When the hatchling molts to the second post-embryonic stage (POII), the DPS differentiates into the lateral (LPZ) and medial (MPZ) proliferative zones. The LPZ and MPZ are characterized by a high number of mitotically active cells from the beginning of post-embryonic life; in contrast, the developing niche contains only very few dividing cells, a characteristic that persists in the adult organism.
Conclusions: Our data suggest that the LPZ and MPZ are largely responsible for the production of new neurons in the early post-embryonic stages, and that the neurogenic niche in the beginning plays a subordinate role. However, as the neuroblasts in the proliferation zones disappear during early post-embryonic life, the neuronal precursors in the niche gradually become the dominant and only mechanism for the generation of new neurons in the adult brain.
Figures












Similar articles
-
First-generation neuronal precursors in the crayfish brain are not self-renewing.Int J Dev Neurosci. 2013 Nov;31(7):657-66. doi: 10.1016/j.ijdevneu.2012.11.010. Epub 2012 Dec 5. Int J Dev Neurosci. 2013. PMID: 23219763 Free PMC article. Review.
-
Primary neuronal precursors in adult crayfish brain: replenishment from a non-neuronal source.BMC Neurosci. 2011 Jun 2;12:53. doi: 10.1186/1471-2202-12-53. BMC Neurosci. 2011. PMID: 21635768 Free PMC article.
-
Cellular basis of neurogenesis in the brain of crayfish, Procambarus clarkii: Neurogenic complex in the olfactory midbrain from hatchlings to adults.Arthropod Struct Dev. 2009 Jul;38(4):339-60. doi: 10.1016/j.asd.2008.12.004. Epub 2009 Feb 23. Arthropod Struct Dev. 2009. PMID: 19185059
-
Adult neurogenesis in crayfish: Origin, expansion, and migration of neural progenitor lineages in a pseudostratified neuroepithelium.J Comp Neurol. 2020 Jun 15;528(9):1459-1485. doi: 10.1002/cne.24820. Epub 2019 Dec 17. J Comp Neurol. 2020. PMID: 31743442
-
Development, growth, and plasticity in the crayfish olfactory system.Microsc Res Tech. 2003 Feb 15;60(3):266-77. doi: 10.1002/jemt.10266. Microsc Res Tech. 2003. PMID: 12539157 Review.
Cited by
-
Adult neurogenesis: ultrastructure of a neurogenic niche and neurovascular relationships.PLoS One. 2012;7(6):e39267. doi: 10.1371/journal.pone.0039267. Epub 2012 Jun 19. PLoS One. 2012. PMID: 22723980 Free PMC article.
-
From Blood to Brain: Adult-Born Neurons in the Crayfish Brain Are the Progeny of Cells Generated by the Immune System.Front Neurosci. 2017 Dec 7;11:662. doi: 10.3389/fnins.2017.00662. eCollection 2017. Front Neurosci. 2017. PMID: 29270102 Free PMC article. Review.
-
First-generation neuronal precursors in the crayfish brain are not self-renewing.Int J Dev Neurosci. 2013 Nov;31(7):657-66. doi: 10.1016/j.ijdevneu.2012.11.010. Epub 2012 Dec 5. Int J Dev Neurosci. 2013. PMID: 23219763 Free PMC article. Review.
-
The 'ventral organs' of Pycnogonida (Arthropoda) are neurogenic niches of late embryonic and post-embryonic nervous system development.PLoS One. 2014 Apr 15;9(4):e95435. doi: 10.1371/journal.pone.0095435. eCollection 2014. PLoS One. 2014. PMID: 24736377 Free PMC article.
-
Timelines in the insect brain: fates of identified neural stem cells generating the central complex in the grasshopper Schistocerca gregaria.Dev Genes Evol. 2014 Feb;224(1):37-51. doi: 10.1007/s00427-013-0462-8. Epub 2013 Dec 17. Dev Genes Evol. 2014. PMID: 24343526
References
-
- Harzsch S, Miller J, Benton J, Dawirs R, Beltz B. Development of the thoracic neuromeres in two crustaceans with different styles of metamorphic development. J Exp Biol. 1998;210:2465–2479. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

