Effects of paliperidone extended release on the symptoms and functioning of schizophrenia
- PMID: 22225965
- PMCID: PMC3282633
- DOI: 10.1186/1472-6904-12-1
Effects of paliperidone extended release on the symptoms and functioning of schizophrenia
Abstract
Background: We aimed to explore relations between symptomatic remission and functionality evaluation in schizophrenia patients treated with paliperidone extended-release (ER), as seen in a normal day-to-day practice, using flexible dosing regimens of paliperidone ER. We explored symptomatic remission rate in patients treated with flexibly dosed paliperidone ER by 8 items of Positive and Negative Syndrome Scale (PANSS) and change of Personal and Social Performance (PSP) scale.
Method: This was a 12-week multicenter, open-label, prospective clinical study conducted in in-patient and out-patient populations. Flexible dosing in the range 3-12 mg/day was used throughout the study. All subjects attended clinic visits on weeks 0, 4, 8, and 12 as usual clinical practice for the 12-week observation period. Data were summarized with respect to demographic and baseline characteristics, efficacy measurement with PANSS scale, PSP, and social functioning score, and safety observations. Descriptive statistics were performed to identify the retention rate at each visit as well as the symptomatic remission rate. Summary statistics of average doses the subjects received were based on all subjects participating in the study.
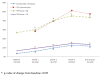
Results: A total of 480 patients were enrolled. Among them, 426 patients (88.8%) had evaluation at week 4 and 350 (72.9%) completed the 12-week evaluation. Patients with at least moderate severity of schizophrenia were evaluated as "mild" or better on PANSS scale by all 8 items after 12 weeks of treatment with paliperidone ER. There was significant improvement in patients' functionality as measured by PSP improvement and score changes. Concerning the other efficacy parameters, PANSS total scale, PSP total scale, and social functioning total scale at the end of study all indicated statistically significant improvement by comparison with baseline. The safety profile also demonstrated that paliperidone ER was well-tolerated without clinically significant changes after treatment administration.
Conclusions: Although the short-term nature of this study may limit the potential for assessing improvements in function, it is noteworthy that in the present short-term study significant improvements in patient personal and social functioning with paliperidone ER treatment were observed, as assessed by PSP scale.
Trial registration: Clinical Trials. PAL-TWN-MA3.
Figures



Similar articles
-
Measuring social functioning with the personal and social performance scale in patients with acute symptoms of schizophrenia: interpretation of results of a pooled analysis of three Phase III trials of paliperidone extended-release tablets.Clin Ther. 2010 Feb;32(2):275-92. doi: 10.1016/j.clinthera.2010.02.003. Clin Ther. 2010. PMID: 20206786
-
Paliperidone extended release: a review of its use in the management of schizophrenia.Drugs. 2010 Jul 9;70(10):1295-317. doi: 10.2165/11204840-000000000-00000. Drugs. 2010. PMID: 20568835 Review.
-
Switching from oral risperidone to flexibly dosed oral paliperidone extended-release: core symptoms, satisfaction, and quality of life in patients with stable but symptomatic schizophrenia: the RISPALI study.Curr Med Res Opin. 2014 Apr;30(4):695-709. doi: 10.1185/03007995.2013.869201. Epub 2013 Dec 16. Curr Med Res Opin. 2014. PMID: 24289141 Clinical Trial.
-
Efficacy and safety of paliperidone extended-release in schizophrenia patients with prominent affective symptoms.J Affect Disord. 2010 Jan;120(1-3):193-9. doi: 10.1016/j.jad.2009.05.025. J Affect Disord. 2010. PMID: 19552963 Clinical Trial.
-
Paliperidone extended release: in adolescents with schizophrenia.Paediatr Drugs. 2012 Dec 1;14(6):417-27. doi: 10.2165/11209900-000000000-00000. Paediatr Drugs. 2012. PMID: 23050744 Review.
Cited by
-
Time to First Line Antiretroviral Therapy Adverse Drug Reaction and its Predictors Among Adult HIV/AIDS Patients on Treatment in Eastern Ethiopia.Front Pharmacol. 2022 Aug 15;13:922744. doi: 10.3389/fphar.2022.922744. eCollection 2022. Front Pharmacol. 2022. PMID: 36046817 Free PMC article.
-
The impact of newer atypical antipsychotics on patient-reported outcomes in schizophrenia.CNS Drugs. 2013 Aug;27(8):625-36. doi: 10.1007/s40263-013-0070-1. CNS Drugs. 2013. PMID: 23757184 Review.
-
A Correlative Classification Study of Schizophrenic Patients with Results of Clinical Evaluation and Structural Magnetic Resonance Images.Behav Neurol. 2016;2016:7849526. doi: 10.1155/2016/7849526. Epub 2016 Oct 24. Behav Neurol. 2016. PMID: 27843197 Free PMC article.
-
Efficacy and tolerability of paliperidone ER in patients with unsatisfactorily controlled schizophrenia by other antipsychotics: a flexible-dose approach.Int Clin Psychopharmacol. 2015 Nov;30(6):329-37. doi: 10.1097/YIC.0000000000000092. Int Clin Psychopharmacol. 2015. PMID: 26230269 Free PMC article. Clinical Trial.
-
Paliperidone Protects SH-SY5Y Cells Against MK-801-Induced Neuronal Damage Through Inhibition of Ca(2+) Influx and Regulation of SIRT1/miR-134 Signal Pathway.Mol Neurobiol. 2016 May;53(4):2498-509. doi: 10.1007/s12035-015-9217-z. Epub 2015 Jun 9. Mol Neurobiol. 2016. PMID: 26055227
References
-
- World Health Organization. Mental health, Disorder management. 2006. http://www.who.int/mental_health/management/schizophrenia/en/
-
- Keshavan MS, Schooler NR. First-episode studies in schizophrenia: criteria and characterization. Schizophr Bull. 1992;18:491–513. - PubMed
-
- Jeffrey Lieberman A, Diana Perkinsa, Aysenil Belger, Miranda Chakos, Fred Jarskog, Kalina Boteva, John Gilmore. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biol Psychiatry. 2001;50:884–97. doi: 10.1016/S0006-3223(01)01303-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

