Precision, accuracy, and user acceptance of the OneTouch SelectSimple blood glucose monitoring system
- PMID: 22226284
- PMCID: PMC3262733
- DOI: 10.1177/193229681100500638
Precision, accuracy, and user acceptance of the OneTouch SelectSimple blood glucose monitoring system
Abstract
Background: The OneTouch® SelectSimple™ blood glucose monitoring system (BGMS) is a device for self-monitoring of blood glucose designed for ease of use. Alarms alert subjects to low [20-69 mg/dl (1.1-3.8 mmol/liter)], high [180-239 mg/dl (9.9-13.2 mmol/liter)], and very high [240-600 mg/dl (13.3-33.1 mmol/liter)] blood glucose readings.
Methods: Repeatability in blood and intermediate precision with aqueous controls were examined using blood from one donor adjusted to different glucose concentrations, and tested with 10 meters and 1 test-strip lot. System accuracy was evaluated with blood samples from 100 diabetes patients tested on 3 test-strip lots, compared with a reference system (YSI 2300 STAT). To test user accuracy, patients (n = 156) and health care professionals (HCPs) tested subject blood with the SelectSimple twice. Health care professionals evaluated subject BGMS technique after a 3-5 day home-testing period. Users evaluated the instructions for use and responded to a user acceptance questionnaire.
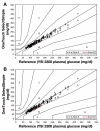
Results: In repeatability and intermediate precision testing, the SelectSimple BGMS had a coefficient of variation of ≤ 5% or standard deviation of ≤ 5 mg/dl. In the clinical accuracy study, 100% of measurements <75 mg/dl (4.2 mmol/liter) were within ± 15 mg/dl (0.8 mmol/liter) of reference value, and 99.6% of measurements ≥ 75 mg/dl (4.2 mmol/liter) were within ±20%. Patients were able to use the BGMS appropriately and evaluated it as easy to use. Acceptance of the SelectSimple BGMS was within predefined limits.
Conclusions: In these studies, the SelectSimple BGMS met all criteria for precision, system, and user accuracy, was easy to use, and was well accepted by patients.
© 2011 Diabetes Technology Society.
Figures




Comment in
-
Analysis of the performance of the OneTouch SelectSimple blood glucose monitoring system: why ease of use studies need to be part of accuracy studies.J Diabetes Sci Technol. 2011 Nov 1;5(6):1610-1. doi: 10.1177/193229681100500639. J Diabetes Sci Technol. 2011. PMID: 22226285 Free PMC article.
References
-
- International Diabetes Federation. IDF Diabetes Atlas. Diabetes and Impaired Glucose Tolerance: Global Burden: Prevalence and Projections, 2010 and 2030. http://www.diabetesatlas.org/content/diabetes-and-impaired-glucose-toler.... Accessed March 24, 2011.
-
- International Diabetes Federation. IDF Diabetes Atlas. Morbidity and Mortality. http://www.diabetesatlas.org/content/diabetes-mortality. Accessed March 24, 2011.
-
- Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301(20):2129–2140. - PubMed
-
- Gupta R, Kumar P. Global diabetes landscape—type 2 diabetes mellitus in South Asia: epidemiology, risk factors, and control. Insulin. 2008;3(2):78–94.
-
- Pan XR, Yang WY, Li GW, Liu J. Prevalence of diabetes and its risk factors in China, 1994. National Diabetes Prevention and Control Cooperative Group. Diabetes Care. 1997;20(11):1664–1669. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

