Prehospital amiodarone may increase the incidence of acute respiratory distress syndrome among patients at risk
- PMID: 22226422
- PMCID: PMC4349894
- DOI: 10.1016/j.jcrc.2011.10.009
Prehospital amiodarone may increase the incidence of acute respiratory distress syndrome among patients at risk
Abstract
Purpose: Amiodarone has been implicated as a risk factor for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) when used in the hospital. This study aims to estimate whether prehospital amiodarone also increases the risk of ALI/ARDS.
Materials: Adult patients admitted to 22 centers with at least 1 risk factor for developing ALI were recruited. In a secondary analysis of this cohort, the prehospital use of amiodarone was documented on admission, and the patients followed for the primary outcome of ALI and secondary outcomes of ARDS, the need for invasive ventilation, and mortality. Dose/duration of amiodarone therapy was not available. Propensity matching was performed to account for imbalances in being assigned to amiodarone. The adjusted risk for ALI/ARDS was then estimated from a conditional logistic regression model of this propensity-matched set.
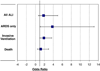
Results: Forty of 5584 patients were on amiodarone at the time of hospitalization; of those, 6 developed ALI, with 5 progressing to ARDS. In comparison, 371 patients not on amiodarone developed ALI, with 224 having ARDS. After propensity score matching, the prehospital use of amiodarone was not statistically associated with an increased risk for all ALI (odds ratio [OR], 1.8; 95% confidence interval [CI], 0.7-5.0; P = .25), invasive ventilation (OR, 1.9; 95% CI, 1.0-3.6; P = .059), or in-hospital mortality (OR, 1.2; 95% CI, 0.5-2.9; P = .75); but its use appeared to significantly increase the risk for ARDS (OR 3.8; 95% CI, 1.1-13.1; P = .036).
Conclusions: Prehospital use of amiodarone may independently increase the risk for ARDS in patients who have at least 1 predisposing condition for ALI.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest: none of the authors have conflicts of interest to report.
Figures

References
-
- Papiris SA, Triantafillidou C, Kolilekas L, et al. Amiodarone: review of pulmonary effects and toxicity. Drug Saf. 2010;33(7):539–558. - PubMed
-
- Ashrafian H, Davey P. Is amiodarone an underrecognized cause of acute respiratory failure in the ICU? Chest. 2001;120(1):275–282. - PubMed
-
- Rotmensch HH, Liron M, Tupilski M, et al. Possible association of pneumonitis with amiodarone therapy. Am Heart J. 1980;100(3):412–413. - PubMed
-
- Ott MC, Khoor A, Leventhal JP, et al. Pulmonary toxicity in patients receiving low-dose amiodarone. Chest. 2003;123(2):646–651. - PubMed

