Ferric iron chelation lowers brain iron levels after intracerebral hemorrhage in rats but does not improve outcome
- PMID: 22226595
- PMCID: PMC3848975
- DOI: 10.1016/j.expneurol.2011.12.030
Ferric iron chelation lowers brain iron levels after intracerebral hemorrhage in rats but does not improve outcome
Abstract
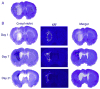
Iron-mediated free radical damage contributes to secondary damage after intracerebral hemorrhage (ICH). Iron is released from heme after hemoglobin breakdown and accumulates in the parenchyma over days and then persists in the brain for months (e.g., hemosiderin). This non-heme iron has been linked to cerebral edema and cell death. Deferoxamine, a ferric iron chelator, has been shown to mitigate iron-mediated damage, but results vary with less protection in the collagenase model of ICH. This study used rapid-scanning X-ray fluorescence (RS-XRF), a synchrotron-based imaging technique, to spatially map total iron and other elements (zinc, calcium and sulfur) at three survival times after collagenase-induced ICH in rats. Total iron was compared to levels of non-heme iron determined by a Ferrozine-based spectrophotometry assay in separate animals. Finally, using RS-XRF we measured iron levels in ICH rats treated with deferoxamine versus saline. The non-heme iron assay showed elevations in injured striatum at 3 days and 4 weeks post-ICH, but not at 1 day. RS-XRF also detected significantly increased iron levels at comparable times, especially notable in the peri-hematoma zone. Changes in other elements were observed in some animals, but these were inconsistent among animals. Deferoxamine diminished total parenchymal iron levels but did not attenuate neurological deficits or lesion volume at 7 days. In summary, ICH significantly increased non-heme and total iron levels. We evaluated the latter and found it to be significantly lowered by deferoxamine, but its failure to attenuate injury or functional impairment in this model raises concern about successful translation to patients.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures






References
-
- Felberg RA, Grotta JC, Shirzadi AL, Strong R, Narayana P, Hill-Felberg SJ, Aronowski J. Cell death in experimental intracerebral hemorrhage: the “black hole” model of hemorrhagic damage. Ann Neurol. 2002;51:517–524. - PubMed
-
- Frantzias J, Sena ES, Macleod MR, Al-Shahi Salman R. Treatment of intracerebral hemorrhage in animal models: meta-analysis. Ann Neurol. 2011;69:389–399. - PubMed
-
- Habib AC, Zheng EM, Haacke ME, Webb RC, Nichol H. Visualizing iron deposition in multiple sclerosis cadaver brains. Am Inst Phys. 2010;1266:78–83.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

