You've come a long way: c-di-GMP signaling
- PMID: 22226607
- PMCID: PMC3320698
- DOI: 10.1016/j.mib.2011.12.008
You've come a long way: c-di-GMP signaling
Abstract
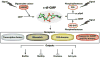
Cyclic dimeric guanosine monophosphate (c-di-GMP) is a common, bacterial second messenger that regulates diverse cellular processes in bacteria. Opposing activities of diguanylate cyclases (DGCs) and phosphodiesterases (PDEs) control c-di-GMP homeostasis in the cell. Many microbes have a large number of genes encoding DGCs and PDEs that are predicted to be part of c-di-GMP signaling networks. Other building blocks of these networks are c-di-GMP receptors which sense the cellular levels of the dinucleotide. C-di-GMP receptors form a more diverse family, including various transcription factors, PilZ domains, degenerate DGCs or PDEs, and riboswitches. Recent studies revealing the molecular basis of c-di-GMP signaling mechanisms enhanced our understanding of how this molecule controls downstream biological processes and how c-di-GMP signaling specificity is achieved.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
References
-
- Christen M, Christen B, Folcher M, Schauerte A, Jenal U. Identification and characterization of a cyclic di-GMP-specific phosphodiesterase and its allosteric control by GTP. J Biol Chem. 2005;280:30829–30837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

