Acquired resistance to drugs targeting receptor tyrosine kinases
- PMID: 22227013
- PMCID: PMC3299940
- DOI: 10.1016/j.bcp.2011.12.025
Acquired resistance to drugs targeting receptor tyrosine kinases
Abstract
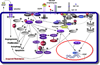
Development of resistance to chemotherapeutic drugs represents a significant hindrance to the effective treatment of cancer patients. The molecular mechanisms responsible have been investigated for over half a century and have revealed the lack of a single cause. Rather, a multitude of mechanisms have been delineated ranging from induction and expression of membrane transporters that pump drugs out of cells (multidrug resistance (MDR) phenotype), changes in the glutathione system and altered metabolism to name a few. Treatment of cancer patients/cancer cells with chemotherapeutic agents and/or molecularly targeted drugs is accompanied by acquisition of resistance to the treatment administered. Chemotherapeutic agent resistance was initially assumed to be due to induction of mutations leading to a resistant phenotype. This has also been true for molecularly targeted drugs. Considerable experience has been gained from the study of agents targeting the Bcr-Abl tyrosine kinase including imatinib, dasatinib and sunitinib. It is clear that mutations alone are not responsible for the many resistance mechanisms in play. Rather, additional mechanisms are involved, ranging from epigenetic changes, alternative splicing and the induction of alternative/compensatory signaling pathways. In this review, resistance to receptor tyrosine kinase inhibitors (RTKIs), RTK-directed antibodies and antibodies that inactivate ligands for RTKs are discussed. New approaches and concepts aimed at avoiding the generation of drug resistance will be examined. The recent observation that many RTKs, including the IGF-1R, are dependence receptors that induce apoptosis in a ligand-independent manner will be discussed and the implications this signaling paradigm has on therapeutic strategies will be considered.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

References
-
- Slomiany MG, Rosenzweig SA. Hypoxia-inducible factor-1-dependent and - independent regulation of insulin-like growth factor-1-stimulated vascular endothelial growth factor secretion. J Pharmacol Exp Ther. 2006;318:666–675. - PubMed
-
- Slomiany MG, Black LA, Kibbey MM, Tingler MA, Day TA, Rosenzweig SA. Insulin-like growth factor-1 receptor and ligand targeting in head and neck squamous cell carcinoma. Cancer Lett. 2007;248:269–279. - PubMed
-
- Rosenzweig SA. Receptor Cross-talk. In: Schwab M, editor. Encyclopedia of Cancer. 2nd Edition. Vol. 3235. Heidelberg: Springer Verlag GmbH; p. 2009.
-
- Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

