Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to camp-mediated effects
- PMID: 22227059
- PMCID: PMC3334479
- DOI: 10.1016/j.expneurol.2011.12.037
Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to camp-mediated effects
Abstract
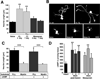
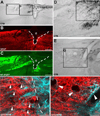
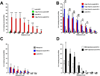
Previous studies indicate that peripheral nerve conditioning lesions significantly enhance central axonal regeneration via modulation of cAMP-mediated mechanisms. To gain insight into the nature and temporal dependence of neural mechanisms underlying conditioning lesion effects on central axonal regeneration, we compared the efficacy of peripheral sciatic nerve crush lesions to cAMP elevations (in lumbar dorsal root ganglia) on central sensory axonal regeneration when administered either before or after cervical spinal cord lesions. We found significantly greater effects of conditioning lesions compared to cAMP elevations on central axonal regeneration when combined with cellular grafts at the lesion site and viral neurotrophin delivery; further, these effects persisted whether conditioning lesions were applied prior to or shortly after spinal cord injury. Indeed, conditioning lesions recruited extensively greater sets of genetic mechanisms of possible relevance to axonal regeneration compared to cAMP administration, and sustained these changes for significantly greater time periods through the post-lesion period. We conclude that cAMP-mediated mechanisms account for only a portion of the potency of conditioning lesions on central axonal regeneration, and that recruitment of broader genetic mechanisms can extend the effect and duration of cellular events that support axonal growth.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


References
-
- Andersen PL, Webber CA, Kimura KA, Schreyer DJ. Cyclic AMP prevents an increase in GAP-43 but promotes neurite growth in cultured adult rat dorsal root ganglion neurons. Exp Neurol. 2000;166:153–165. - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300.
-
- Bethea JR, Dietrich WD. Targeting the host inflammatory response in traumatic spinal cord injury. Curr Opin Neurol. 2002;15:355–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

