Spermine, a molecular switch regulating EGFR, integrin β3, Src, and FAK scaffolding
- PMID: 22227249
- PMCID: PMC3334284
- DOI: 10.1016/j.cellsig.2011.12.016
Spermine, a molecular switch regulating EGFR, integrin β3, Src, and FAK scaffolding
Abstract
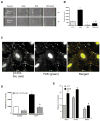
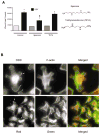
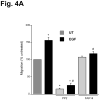
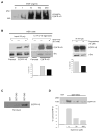
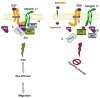
Intracellular polyamine levels are highly regulated by the activity of ornithine decarboxylase (ODC), which catalyzes the first rate-limiting reaction in polyamine biosynthesis, producing putrescine, which is subsequently converted to spermidine and spermine. We have shown that polyamines regulate proliferation, migration, and apoptosis in intestinal epithelial cells. Polyamines regulate key signaling events at the level of the EGFR and Src. However, the precise mechanism of action of polyamines is unknown. In the present study, we demonstrate that ODC localizes in lamellipodia and in adhesion plaques during cell spreading. Spermine regulates EGF-induced migration by modulating the interaction of the EGFR with Src. The EGFR interacted with integrin β3, Src, and focal adhesion kinase (FAK). Active Src (pY418-Src) localized with FAK during spreading and migration. Spermine prevented EGF-induced binding of the EGFR with integrin β3, Src, and FAK. Activation of Src and FAK was necessary for EGF-induced migration in HEK293 cells. EGFR-mediated Src activation in live HEK293 cells using a FRET based Src reporter showed that polyamine depletion significantly increased Src kinase activity. In vitro binding studies showed that spermine directly binds Src, and preferentially interacts with the SH2 domain of Src. The physical interaction between Src and the EGFR was severely attenuated by spermine. Therefore, spermine acts as a molecular switch in regulating EGFR-Src coupling both physically and functionally. Upon activation of the EGFR, integrin β3, FAK and Src are recruited to EGFR leading to the trans-activation of both the EGFR and Src and to the Src-mediated phosphorylation of FAK. The activation of FAK induced Rho-GTPases and subsequently migration. This is the first study to define mechanistically how polyamines modulate Src function at the molecular level.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
EGFR plays a pivotal role in the regulation of polyamine-dependent apoptosis in intestinal epithelial cells.Cell Signal. 2007 Dec;19(12):2519-27. doi: 10.1016/j.cellsig.2007.08.001. Epub 2007 Aug 15. Cell Signal. 2007. PMID: 17825525 Free PMC article.
-
Integrin beta3-mediated Src activation regulates apoptosis in IEC-6 cells via Akt and STAT3.Biochem J. 2006 Aug 1;397(3):437-47. doi: 10.1042/BJ20060256. Biochem J. 2006. PMID: 16669788 Free PMC article.
-
Leu33Pro (PlA) polymorphism of integrin beta3 modulates platelet Src pY418 and focal adhesion kinase pY397 phosphorylation in response to abnormally high shear stress.Blood Coagul Fibrinolysis. 2018 Sep;29(6):488-495. doi: 10.1097/MBC.0000000000000744. Blood Coagul Fibrinolysis. 2018. PMID: 29965811
-
New concepts regarding focal adhesion kinase promotion of cell migration and proliferation.J Cell Biochem. 2006 Sep 1;99(1):35-52. doi: 10.1002/jcb.20956. J Cell Biochem. 2006. PMID: 16823799 Review.
-
Signal Transduction Mechanisms of Focal Adhesions: Src and FAK-Mediated Cell Response.Front Biosci (Landmark Ed). 2024 Nov 20;29(11):392. doi: 10.31083/j.fbl2911392. Front Biosci (Landmark Ed). 2024. PMID: 39614431 Review.
Cited by
-
Biocompatible Cationic Lipoamino Acids as Counterions for Oral Administration of API-Ionic Liquids.Pharm Res. 2022 Oct;39(10):2405-2419. doi: 10.1007/s11095-022-03305-y. Epub 2022 Jun 3. Pharm Res. 2022. PMID: 35661084 Free PMC article.
-
Integrin-associated molecules and signalling cross talking in osteoclast cytoskeleton regulation.J Cell Mol Med. 2020 Mar;24(6):3271-3281. doi: 10.1111/jcmm.15052. Epub 2020 Feb 11. J Cell Mol Med. 2020. PMID: 32045092 Free PMC article. Review.
-
Exercise training preserves ischemic preconditioning in aged rat hearts by restoring the myocardial polyamine pool.Oxid Med Cell Longev. 2014;2014:457429. doi: 10.1155/2014/457429. Epub 2014 Oct 23. Oxid Med Cell Longev. 2014. PMID: 25404991 Free PMC article.
-
A back-door insight into the modulation of Src kinase activity by the polyamine spermidine.Elife. 2023 Jun 30;12:e85872. doi: 10.7554/eLife.85872. Elife. 2023. PMID: 37387273 Free PMC article.
-
The MAP3K ZAK, a novel modulator of ERK-dependent migration, is upregulated in colorectal cancer.Oncogene. 2016 Jun 16;35(24):3190-200. doi: 10.1038/onc.2015.379. Epub 2015 Nov 2. Oncogene. 2016. PMID: 26522728
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

