Complex morphology and functional dynamics of vital murine intestinal mucosa revealed by autofluorescence 2-photon microscopy
- PMID: 22227801
- PMCID: PMC3278620
- DOI: 10.1007/s00418-011-0905-0
Complex morphology and functional dynamics of vital murine intestinal mucosa revealed by autofluorescence 2-photon microscopy
Abstract
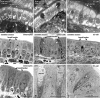
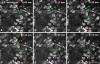
The mucosa of the gastrointestinal tract is a dynamic tissue composed of numerous cell types with complex cellular functions. Study of the vital intestinal mucosa has been hampered by lack of suitable model systems. We here present a novel animal model that enables highly resolved three-dimensional imaging of the vital murine intestine in anaesthetized mice. Using intravital autofluorescence 2-photon (A2P) microscopy we studied the choreographed interactions of enterocytes, goblet cells, enteroendocrine cells and brush cells with other cellular constituents of the small intestinal mucosa over several hours at a subcellular resolution and in three dimensions. Vigorously moving lymphoid cells and their interaction with constituent parts of the lamina propria were examined and quantitatively analyzed. Nuclear and lectin staining permitted simultaneous characterization of autofluorescence and admitted dyes and yielded additional spectral information that is crucial to the interpretation of the complex intestinal mucosa. This novel intravital approach provides detailed insights into the physiology of the small intestine and especially opens a new window for investigating cellular dynamics under nearly physiological conditions.
Figures






References
-
- Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. - PubMed
-
- Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, Bacallao RL, Molitoris BA. Functional studies of the kidney of living animals using multicolour two-photon microscopy. Am J Physiol. 2002;283:905–916. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

