Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up
- PMID: 22228146
- PMCID: PMC3260132
- DOI: 10.1093/jnci/djr500
Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up
Abstract
Background: The prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial was undertaken to determine whether there is a reduction in prostate cancer mortality from screening using serum prostate-specific antigen (PSA) testing and digital rectal examination (DRE). Mortality after 7-10 years of follow-up has been reported previously. We report extended follow-up to 13 years after the trial.
Methods: A total of 76 685 men, aged 55-74 years, were enrolled at 10 screening centers between November 1993 and July 2001 and randomly assigned to the intervention (organized screening of annual PSA testing for 6 years and annual DRE for 4 years; 38 340 men) and control (usual care, which sometimes included opportunistic screening; 38 345 men) arms. Screening was completed in October 2006. All incident prostate cancers and deaths from prostate cancer through 13 years of follow-up or through December 31, 2009, were ascertained. Relative risks (RRs) were estimated as the ratio of observed rates in the intervention and control arms, and 95% confidence intervals (CIs) were calculated assuming a Poisson distribution for the number of events. Poisson regression modeling was used to examine the interactions with respect to prostate cancer mortality between trial arm and age, comorbidity status, and pretrial PSA testing. All statistical tests were two-sided.
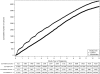
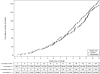
Results: Approximately 92% of the study participants were followed to 10 years and 57% to 13 years. At 13 years, 4250 participants had been diagnosed with prostate cancer in the intervention arm compared with 3815 in the control arm. Cumulative incidence rates for prostate cancer in the intervention and control arms were 108.4 and 97.1 per 10 000 person-years, respectively, resulting in a relative increase of 12% in the intervention arm (RR = 1.12, 95% CI = 1.07 to 1.17). After 13 years of follow-up, the cumulative mortality rates from prostate cancer in the intervention and control arms were 3.7 and 3.4 deaths per 10 000 person-years, respectively, resulting in a non-statistically significant difference between the two arms (RR = 1.09, 95% CI = 0.87 to 1.36). No statistically significant interactions with respect to prostate cancer mortality were observed between trial arm and age (P(interaction) = .81), pretrial PSA testing (P(interaction) = .52), and comorbidity (P(interaction) = .68).
Conclusions: After 13 years of follow-up, there was no evidence of a mortality benefit for organized annual screening in the PLCO trial compared with opportunistic screening, which forms part of usual care, and there was no apparent interaction with age, baseline comorbidity, or pretrial PSA testing.
Figures
Comment in
-
Prostate cancer: PSA screening--more data, more debate.Nat Rev Urol. 2012 Feb 9;9(2):59. doi: 10.1038/nrurol.2012.7. Nat Rev Urol. 2012. PMID: 22318289 No abstract available.
-
Re: prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up.J Natl Cancer Inst. 2012 May 16;104(10):793; author reply 793-4. doi: 10.1093/jnci/djs205. Epub 2012 Apr 5. J Natl Cancer Inst. 2012. PMID: 22491229 No abstract available.
-
Words of wisdom. Re: Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up.Eur Urol. 2012 Aug;62(2):353. doi: 10.1016/j.eururo.2012.05.031. Eur Urol. 2012. PMID: 22748397 No abstract available.
-
Re: Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up.J Urol. 2012 Aug;188(2):429-30. doi: 10.1016/j.juro.2012.04.071. Epub 2012 Jun 14. J Urol. 2012. PMID: 22784722 No abstract available.
-
Commentary on "prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: Mortality results after 13 years of follow-up". Andriole GL, Crawford ED, Grubb RL III, Buys SS, Chia D, Church TR, Fouad MN, Isaacs C, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Ragard LR, Clapp JD, Rathmell JM, Riley TL, Hsing AW, Izmirlian G, Pinsky PF, Kramer BS, Miller AB, Gohagan JK, Prorok PC; PLCO Project Team.Collaborators (18) Buring JE, Alberts D, Carter HB, Chodak G, Hawk E, Malm H, Mayer RJ, Piantadosi S, Silvestri GA, Thompson IM, Westhoff CL, Kahn JP, Levin B, DeMets D, O'Fallon JR, Porter AT, Ashton MM, Black WC, Division of Urologic Surgery, Washington University School of Medicine, St. Louis, MO 63130, USA: J. Natl Cancer Inst 2012; 104(2):125-32. Epub January 6, 2012.Urol Oncol. 2012 Nov-Dec;30(6):960-1. doi: 10.1016/j.urolonc.2012.08.006. Urol Oncol. 2012. PMID: 23218077 No abstract available.
References
-
- Schröder FH, Hugosson J, Roobol MJ, et al. for the ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. New Eng J Med. 2009;360:1320–1328. - PubMed
-
- Catalona WJ. Screening for prostate cancer. New Eng J Med. 2009;361:202. - PubMed
-
- Fleming ID, Cooper JS, Henson DE, et al., editors. AJCC Cancer Staging Manual. 5th ed. Philadelphia, PA: Lippincott-Raven; 1997.
Publication types
MeSH terms
Substances
Grants and funding
- N01-CN-25524/CN/NCI NIH HHS/United States
- N01-CN-25513/CN/NCI NIH HHS/United States
- N01-CN-75022/CN/NCI NIH HHS/United States
- N01-CN-25514/CN/NCI NIH HHS/United States
- N01-CN-25512/CN/NCI NIH HHS/United States
- N01 CN025511/CA/NCI NIH HHS/United States
- N01-CN-25515/CN/NCI NIH HHS/United States
- N01 CN025516/CA/NCI NIH HHS/United States
- N01 CN075022/CA/NCI NIH HHS/United States
- N01 CN025404/CA/NCI NIH HHS/United States
- N01 CN025518/CA/NCI NIH HHS/United States
- N01 CN025515/CA/NCI NIH HHS/United States
- N01-CN-25476/CN/NCI NIH HHS/United States
- N01-CN-25522/CN/NCI NIH HHS/United States
- N01-CN-25511/CN/NCI NIH HHS/United States
- N01 CN025512/CA/NCI NIH HHS/United States
- N01 CN025522/CA/NCI NIH HHS/United States
- N01 CN025513/CA/NCI NIH HHS/United States
- N01 CN025524/CA/NCI NIH HHS/United States
- N01 CN025476/CA/NCI NIH HHS/United States
- N01-CN-25404/CN/NCI NIH HHS/United States
- N01-CN-25516/CN/NCI NIH HHS/United States
- N01-CN-25518/CN/NCI NIH HHS/United States
- N01 CN025514/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

