Gastrin inhibits a novel, pathological colon cancer signaling pathway involving EGR1, AE2, and P-ERK
- PMID: 22228178
- PMCID: PMC4986693
- DOI: 10.1007/s00109-011-0851-2
Gastrin inhibits a novel, pathological colon cancer signaling pathway involving EGR1, AE2, and P-ERK
Abstract
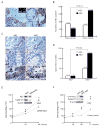
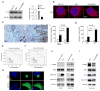
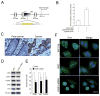
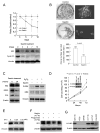
Human anion exchanger 2 (AE2) is a plasma membrane protein that regulates intracellular pH and cell volume. AE2 contributes to transepithelial transport of chloride and bicarbonate in normal colon and other epithelial tissues. We now report that AE2 overexpression in colon cancer cells is correlated with expression of the nuclear proliferation marker, Ki67. Survival analysis of 24 patients with colon cancer in early stage or 33 patients with tubular adenocarcinoma demonstrated that expression of AE2 is correlated with poor prognosis. Cellular and molecular experiments indicated that AE2 expression promoted proliferation of colon cancer cells. In addition, we found that transcription factor EGR1 underlies AE2 upregulation and the AE2 sequester p16INK4a (P16) in the cytoplasm of colon cancer cells. Cytoplasmic P16 enhanced ERK phosphorylation and promoted proliferation of colon cancer cells. Gastrin inhibited proliferation of colon cancer cells by suppressing expression of EGR1 and AE2 and by blocking ERK phosphorylation. Taken together, our data describe a novel EGR1/AE2/P16/P-ERK signaling pathway in colon carcinogenesis, with implications for pathologic prognosis and for novel therapeutic approaches.
Conflict of interest statement
Figures

Similar articles
-
EGR1 is critical for gastrin-dependent upregulation of anion exchanger 2 in gastric cancer cells.FEBS J. 2013 Jan;280(1):174-83. doi: 10.1111/febs.12058. Epub 2012 Nov 29. FEBS J. 2013. PMID: 23121767
-
Up-regulation of microRNA 506 leads to decreased Cl-/HCO3- anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis.Hepatology. 2012 Aug;56(2):687-97. doi: 10.1002/hep.25691. Epub 2012 Jul 10. Hepatology. 2012. PMID: 22383162 Free PMC article.
-
Expression of AE1/p16 promoted degradation of AE2 in gastric cancer cells.BMC Cancer. 2016 Sep 5;16(1):716. doi: 10.1186/s12885-016-2751-x. BMC Cancer. 2016. PMID: 27595783 Free PMC article.
-
Molecular physiology of SLC4 anion exchangers.Exp Physiol. 2006 Jan;91(1):153-61. doi: 10.1113/expphysiol.2005.031765. Epub 2005 Oct 20. Exp Physiol. 2006. PMID: 16239253 Review.
-
Role of the anion exchanger 2 in the pathogenesis and treatment of primary biliary cirrhosis.Dig Dis. 2011;29(1):103-12. doi: 10.1159/000324144. Epub 2011 Jun 17. Dig Dis. 2011. PMID: 21691115 Review.
Cited by
-
Potential Theranostic Roles of SLC4 Molecules in Human Diseases.Int J Mol Sci. 2023 Oct 13;24(20):15166. doi: 10.3390/ijms242015166. Int J Mol Sci. 2023. PMID: 37894847 Free PMC article. Review.
-
Trafficking of carbonic anhydrase 12 and bicarbonate transporters by histamine stimulation mediates intracellular acidic scenario in lung cancer cells.J Enzyme Inhib Med Chem. 2023 Dec;38(1):2247181. doi: 10.1080/14756366.2023.2247181. J Enzyme Inhib Med Chem. 2023. PMID: 37587861 Free PMC article.
-
ALDH1B1 Is Crucial for Colon Tumorigenesis by Modulating Wnt/β-Catenin, Notch and PI3K/Akt Signaling Pathways.PLoS One. 2015 May 7;10(5):e0121648. doi: 10.1371/journal.pone.0121648. eCollection 2015. PLoS One. 2015. PMID: 25950950 Free PMC article.
-
Chloride Channels and Transporters: Roles beyond Classical Cellular Homeostatic pH or Ion Balance in Cancers.Cancers (Basel). 2022 Feb 9;14(4):856. doi: 10.3390/cancers14040856. Cancers (Basel). 2022. PMID: 35205604 Free PMC article. Review.
-
Effect of Proton Pump Inhibitors on Colorectal Cancer.Int J Mol Sci. 2020 May 29;21(11):3877. doi: 10.3390/ijms21113877. Int J Mol Sci. 2020. PMID: 32485921 Free PMC article.
References
-
- She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, Solit DB, Rosen N. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. S1535-6108(10)00235-7 [pii] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

