White spotting variant mouse as an experimental model for ovarian aging and menopausal biology
- PMID: 22228319
- PMCID: PMC3326177
- DOI: 10.1097/gme.0b013e318239cc53
White spotting variant mouse as an experimental model for ovarian aging and menopausal biology
Abstract
Objective: Menopause is a unique phenomenon in modern women, as most mammalian species possess a reproductive period comparable with their life span. Menopause is caused by the depletion of germ cell-containing ovarian follicles and in laboratory studies is usually modeled in animals in which the ovarian function is removed through ovariectomy or chemical poisoning of the germ cells. Our objective was to explore and characterize the white spotting variant (Wv) mice that have reduced ovarian germ cell abundance, a result of a point mutation in the c-kit gene that decreases kinase activity, as a genetic model for use in menopause studies.
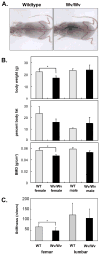
Methods: Physiological and morphological features associated with menopause were determined in female Wv/Wv mice compared with age-matched wildtype controls. Immunohistochemistry was used to evaluate the presence and number of follicles in paraffin-embedded ovaries. Bone density and body composition were evaluated using the PIXImus x-ray densitometer, and lipids, calcium, and hormone levels were determined in serum using antigen-specific enzyme immunoassays. Heart and body weight were measured, and cardiac function was evaluated using transthoracic echocardiography.
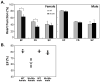
Results: The ovaries of the Wv/Wv females have a greatly reduced number of normal germ cells at birth compared with wildtype mice. The remaining follicles are depleted by around 2 months, and the ovaries develop benign epithelial lesions that resemble morphological changes that occur during ovarian aging, whereas a normal mouse ovary has numerous follicles at all stages of development and retains some follicles even in advanced age. Wv mice have elevated plasma gonadotropins and reduced estrogen and progesterone levels, a significant reduction in bone mass density, and elevated serum cholesterol and lipoprotein levels. Moreover, the Wv female mice have enlarged hearts and reduced cardiac function.
Conclusions: The reduction of c-kit activity in Wv mice leads to a substantially diminished follicular endowment in newborn mice and premature depletion of follicles in young mice, although mutant females have a normal life span after cessation of ovarian function. The Wv female mice exhibit consistent physiological changes that resemble common features of postmenopausal women. These alterations include follicle depletion, morphological aging of the ovary, altered serum levels of cholesterol, gonadotropins and steroid hormones, decreased bone density, and reduced cardiac function. These changes were not observed in male mice, either age-matched male Wv/Wv or wildtype mice, and are improbably caused by global loss of c-kit function. The Wv mouse may be a genetic, intact-ovary model that mimics closely the phenotypes of human menopause to be used for further studies to understand the mechanisms of menopausal biology.
Conflict of interest statement
Figures


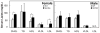

Similar articles
-
Global deletion of Trp53 reverts ovarian tumor phenotype of the germ cell-deficient white spotting variant (Wv) mice.Neoplasia. 2015 Jan;17(1):89-100. doi: 10.1016/j.neo.2014.11.005. Neoplasia. 2015. PMID: 25622902 Free PMC article.
-
Development of a mouse model of menopausal ovarian cancer.Front Oncol. 2014 Feb 26;4:36. doi: 10.3389/fonc.2014.00036. eCollection 2014. Front Oncol. 2014. PMID: 24616881 Free PMC article. Review.
-
Reduction in minipubertal gonadotropin levels alters reproductive lifespan and ovarian follicular loss in female mice.Hum Reprod. 2025 Apr 1;40(4):717-729. doi: 10.1093/humrep/deaf019. Hum Reprod. 2025. PMID: 39948193 Free PMC article.
-
Oocyte growth and follicular development in KIT-deficient Fas-knockout mice.Reproduction. 2007 Jan;133(1):117-25. doi: 10.1530/REP-06-0161. Reproduction. 2007. PMID: 17244738
-
Follicular depletion during the menopausal transition.Ann N Y Acad Sci. 1990;592:13-20; discussion 44-51. doi: 10.1111/j.1749-6632.1990.tb30312.x. Ann N Y Acad Sci. 1990. PMID: 2197939 Review.
Cited by
-
High Doses of D-Chiro-Inositol Alone Induce a PCO-Like Syndrome and Other Alterations in Mouse Ovaries.Int J Mol Sci. 2021 May 26;22(11):5691. doi: 10.3390/ijms22115691. Int J Mol Sci. 2021. PMID: 34073634 Free PMC article.
-
Activation of Notch Signaling by Oocytes and Jag1 in Mouse Ovarian Granulosa Cells.Endocrinology. 2019 Dec 1;160(12):2863-2876. doi: 10.1210/en.2019-00564. Endocrinology. 2019. PMID: 31609444 Free PMC article.
-
Prediction of ovarian aging using ovarian expression of BMP15, GDF9, and C-KIT.Exp Biol Med (Maywood). 2020 Apr;245(8):711-719. doi: 10.1177/1535370220915826. Epub 2020 Mar 29. Exp Biol Med (Maywood). 2020. PMID: 32223330 Free PMC article.
-
Follicle Depletion Provides a Permissive Environment for Ovarian Carcinogenesis.Mol Cell Biol. 2016 Aug 26;36(18):2418-30. doi: 10.1128/MCB.00202-16. Print 2016 Sep 15. Mol Cell Biol. 2016. PMID: 27354067 Free PMC article.
-
Cyclooxygenase-1 inhibition prolongs postnatal ovarian follicle lifespan in mice.Biol Reprod. 2013 Oct 31;89(4):103. doi: 10.1095/biolreprod.113.111070. Print 2013 Oct. Biol Reprod. 2013. PMID: 23966321 Free PMC article.
References
-
- Finn CA. Reproductive ageing and the menopause. Int J Dev Biol. 2001;45:613–617. - PubMed
-
- Gao J, Tiwari-Pandey R, Samadfam R, Yang Y, Miao D, Karaplis AC, Sairam MR, Goltzman D. Altered ovarian function affects skeletal homeostasis independent of the action of follicle-stimulating hormone. Endocrinology. 2007;148:2613–21. - PubMed
-
- Erickson GF. Ovarian anatomy and physiology. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2000. pp. 13–31.
-
- Gold EB. Demographics, environmental influences, and ethnic and international differences in the menopausal experience. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2000. pp. 189–201.
-
- Gosden RG, editor. Biology of Menopause: The causes and consequences of ovarian ageing. London: Academic Press; 1985.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

