CD40-targeted adenoviral cancer vaccines: the long and winding road to the clinic
- PMID: 22228547
- PMCID: PMC3433169
- DOI: 10.1002/jgm.1648
CD40-targeted adenoviral cancer vaccines: the long and winding road to the clinic
Abstract
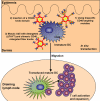
The ability of dendritic cells (DCs) to orchestrate innate and adaptive immune responses has been exploited to develop potent anti-cancer immunotherapies. Recent clinical trials exploring the efficacy of ex vivo modified autologous DC-based vaccines have reported some promising results. However, in vitro generation of autologous DCs for clinical administration, their loading with tumor associated antigens (TAAs) and their activation, is laborious and expensive, and, as a result of inter-individual variability in the personalized vaccines, remains poorly standardized. An attractive alternative approach is to load resident DCs in vivo by targeted delivery of TAAs, using viral vectors and activating them simultaneously. To this end, we have constructed genetically-modified adenoviral (Ad) vectors and bispecific adaptor molecules to retarget Ad vectors encoding TAAs to the CD40 receptor on DCs. Pre-clinical human and murine studies conducted so far have clearly demonstrated the suitability of a 'two-component' (i.e. Ad and adaptor molecule) configuration for targeted modification of DCs in vivo for cancer immunotherapy. This review summarizes recent progress in the development of CD40-targeted Ad-based cancer vaccines and highlights pre-clinical issues in the clinical translation of this approach.
Copyright © 2012 John Wiley & Sons, Ltd.
Figures



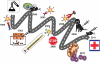
Comment in
-
When transgenes shape immunity: cancer immune-gene therapy.J Gene Med. 2012 Jun;14(6):384-5. doi: 10.1002/jgm.2645. J Gene Med. 2012. PMID: 22736622 No abstract available.
References
-
- Tacken PJ, de Vries IJ, Torensma R, et al. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. - PubMed
-
- Wei H, Wang S, Zhang D, et al. Targeted delivery of tumor antigens to activated dendritic cells via CD11c molecules induces potent antitumor immunity in mice. Clin Cancer Res. 2009;15:4612–4621. - PubMed
-
- Dickgreber N, Stoitzner P, Bai Y, et al. Targeting antigen to MHC class II molecules promotes efficient cross-presentation and enhances immunotherapy. J Immunol. 2009;182:1260–1269. - PubMed
-
- Tagliani E, Guermonprez P, Sepulveda J, et al. Selection of an antibody library identifies a pathway to induce immunity by targeting CD36 on steady-state CD8 alpha+ dendritic cells. J Immunol. 2008;180:3201–3209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

