REST regulates oncogenic properties of glioblastoma stem cells
- PMID: 22228704
- PMCID: PMC4039365
- DOI: 10.1002/stem.1020
REST regulates oncogenic properties of glioblastoma stem cells
Erratum in
- Stem Cells. 2012 May;30(5):1049
Abstract
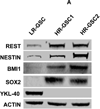
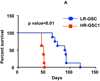
Glioblastoma multiforme (GBM) tumors are the most common malignant primary brain tumors in adults. Although many GBM tumors are believed to be caused by self-renewing, glioblastoma-derived stem-like cells (GSCs), the mechanisms that regulate self-renewal and other oncogenic properties of GSCs are only now being unraveled. Here we showed that GSCs derived from GBM patient specimens express varying levels of the transcriptional repressor repressor element 1 silencing transcription factor (REST), suggesting heterogeneity across different GSC lines. Loss- and gain-of-function experiments indicated that REST maintains self-renewal of GSCs. High REST-expressing GSCs (HR-GSCs) produced tumors histopathologically distinct from those generated by low REST-expressing GSCs (LR-GSCs) in orthotopic mouse brain tumor models. Knockdown of REST in HR-GSCs resulted in increased survival in GSC-transplanted mice and produced tumors with higher apoptotic and lower invasive properties. Conversely, forced expression of exogenous REST in LR-GSCs produced decreased survival in mice and produced tumors with lower apoptotic and higher invasive properties, similar to HR-GSCs. Thus, based on our results, we propose that a novel function of REST is to maintain self-renewal and other oncogenic properties of GSCs and that REST can play a major role in mediating tumorigenicity in GBM.
Copyright © 2011 AlphaMed Press.
Figures





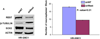
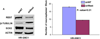
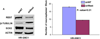











Similar articles
-
REST represses miR-124 and miR-203 to regulate distinct oncogenic properties of glioblastoma stem cells.Neuro Oncol. 2017 Apr 1;19(4):514-523. doi: 10.1093/neuonc/now232. Neuro Oncol. 2017. PMID: 28040710 Free PMC article.
-
REST-DRD2 mechanism impacts glioblastoma stem cell-mediated tumorigenesis.Neuro Oncol. 2019 Jun 10;21(6):775-785. doi: 10.1093/neuonc/noz030. Neuro Oncol. 2019. PMID: 30953587 Free PMC article.
-
Engagement of cellular prion protein with the co-chaperone Hsp70/90 organizing protein regulates the proliferation of glioblastoma stem-like cells.Stem Cell Res Ther. 2017 Apr 17;8(1):76. doi: 10.1186/s13287-017-0518-1. Stem Cell Res Ther. 2017. PMID: 28412969 Free PMC article.
-
The Importance of Tumor Stem Cells in Glioblastoma Resistance to Therapy.Int J Mol Sci. 2021 Apr 8;22(8):3863. doi: 10.3390/ijms22083863. Int J Mol Sci. 2021. PMID: 33917954 Free PMC article. Review.
-
Off the Clock: the Non-canonical Roles of Cyclin-Dependent Kinases in Neural and Glioma Stem Cell Self-Renewal.Mol Neurobiol. 2022 Nov;59(11):6805-6816. doi: 10.1007/s12035-022-03009-9. Epub 2022 Aug 31. Mol Neurobiol. 2022. PMID: 36042143 Review.
Cited by
-
Feedback circuitry between miR-218 repression and RTK activation in glioblastoma.Sci Signal. 2015 May 5;8(375):ra42. doi: 10.1126/scisignal.2005978. Sci Signal. 2015. PMID: 25943352 Free PMC article.
-
Inhibition of REST Suppresses Proliferation and Migration in Glioblastoma Cells.Int J Mol Sci. 2016 May 3;17(5):664. doi: 10.3390/ijms17050664. Int J Mol Sci. 2016. PMID: 27153061 Free PMC article.
-
The tumor suppressor microRNA, miR-124a, is regulated by epigenetic silencing and by the transcriptional factor, REST in glioblastoma.Tumour Biol. 2014 Feb;35(2):1459-65. doi: 10.1007/s13277-013-1200-6. Epub 2013 Sep 26. Tumour Biol. 2014. PMID: 24068568
-
Selective repression of gene expression in neuropathic pain by the neuron-restrictive silencing factor/repressor element-1 silencing transcription (NRSF/REST).Neurosci Lett. 2016 Jun 20;625:20-5. doi: 10.1016/j.neulet.2015.12.003. Epub 2015 Dec 8. Neurosci Lett. 2016. PMID: 26679228 Free PMC article. Review.
-
Targeting of REST with rationally-designed small molecule compounds exhibits synergetic therapeutic potential in human glioblastoma cells.BMC Biol. 2024 Apr 12;22(1):83. doi: 10.1186/s12915-024-01879-0. BMC Biol. 2024. PMID: 38609948 Free PMC article.
References
-
- Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10:459–466. - PubMed
-
- Gilbertson RJ, Gutmann DH. Tumorigenesis in the brain: location, location, location. Cancer Res. 2007;67:5579–5582. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

