Recovery from glycerol-induced acute kidney injury is accelerated by suramin
- PMID: 22228809
- PMCID: PMC3310704
- DOI: 10.1124/jpet.111.190249
Recovery from glycerol-induced acute kidney injury is accelerated by suramin
Abstract
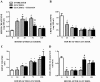
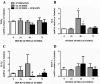
Acute kidney injury (AKI) is a common and potentially life-threatening complication after ischemia/reperfusion and exposure to nephrotoxic agents. In this study, we examined the efficacy and mechanism(s) of suramin in promoting recovery from glycerol-induced AKI, a model of rhabdomyolysis-induced AKI. After intramuscular glycerol injection (10 ml of 50% glycerol per kilogram) into male Sprague-Dawley rats, serum creatinine maximally increased at 24 to 72 h and then decreased at 120 h. Creatinine clearance (CrCl) decreased 75% at 24 to 72 h and increased at 120 h. Suramin (1 mg/kg i.v.) administered 24 h after glycerol accelerated recovery of renal function as demonstrated by increased CrCl, decreased renal kidney injury molecule-1, and improved histopathology 72 h after glycerol injection. Suramin treatment decreased interleukin-1β (IL-1β) mRNA, transforming growth factor-β(1) (TGF-β(1)), phospho-p65 of nuclear factor-κB (NF-κB), and cleaved caspase-3 at 48 h compared with glycerol alone. Suramin treatment also decreased glycerol-induced activation of intracellular adhesion molecule-1 (ICAM-1) and leukocyte infiltration at 72 h. Urinary/renal neutrophil gelatinase-associated lipocalin 2 (NGAL) levels, hemeoxygenase-1 expression, and renal cell proliferation were increased by suramin compared with glycerol alone at 72 h. Mechanistically, suramin decreases early glycerol-induced proinflammatory (IL-1β and NF-κB) and growth inhibitory (TGF-β(1)) mediators, resulting in the prevention of late downstream inflammatory effects (ICAM-1 and leukocyte infiltration) and increasing compensatory nephrogenic repair. These results support the hypothesis that delayed administration of suramin is effective in abrogating apoptosis, attenuating inflammation, and enhancing nephrogenic repair after glycerol-induced AKI.
Figures









References
-
- Bagley WH, Yang H, Shah KH. (2007) Rhabdomyolysis. Intern Emerg Med 2:210–218 - PubMed
-
- Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. (2002) Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem 277:39739–39748 - PubMed
-
- Bates CM, Lin F. (2005) Future strategies in the treatment of acute renal failure: growth factors, stem cells, and other novel therapies. Curr Opin Pediatr 17:215–220 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

