Enhanced enzyme activity through electron transfer between single-walled carbon nanotubes and horseradish peroxidase
- PMID: 22228910
- PMCID: PMC3249833
- DOI: 10.1016/j.carbon.2011.10.053
Enhanced enzyme activity through electron transfer between single-walled carbon nanotubes and horseradish peroxidase
Abstract
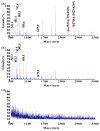
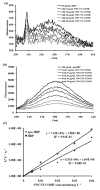
Better understanding of electron transfer (ET) taking place at the nano-bio interface can guide design of more effective functional materials used in fuel cells, biosensors, and medical devices. Single-walled carbon nanotube (SWCNT) coupled with biological enzymes serves as a model system for studying the ET mechanism, as demonstrated in the present study. SWCNT enhanced the activity of horseradish peroxidase (HRP) in the solution-based redox reaction by binding to HRP at a site proximate to the enzyme's activity center and participating in the ET process. ET to and from SWCNT was clearly observable using near-infrared spectroscopy. The capability of SWCNT in receiving electrons and the direct attachment of HRP to the surface of SWCNT strongly affected the enzyme activity due to the direct involvement of SWCNT in ET.
Figures




References
-
- Wang J. Carbon-nanotube based electrochemical biosensors: a review. Electroanal. 2005;17(1):7–14.
-
- Willner B, Katz E, Willner I. Electrical contacting of redox proteins by nanotechnological means. Curr Opin Biotechnol. 2006;17(6):589–96. - PubMed
-
- Willner I, Baron R, Willner B. Integrated nanoparticle-biomolecule systems for biosensing and bioelectronics. Biosens & Bioelectron. 2007;22(9–10):1841–52. - PubMed
-
- Tsai T, Heckert G, Neves LF, Tan Y, Kao D, Harrison RG, et al. Adsorption of glucose oxidase onto single-walled carbon nanotubes and its application in layer-by-layer biosensors. Anal Chem. 2009;81(19):7917–25. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
