Randomised, double blind, placebo-controlled trial of echinacea supplementation in air travellers
- PMID: 22229040
- PMCID: PMC3249603
- DOI: 10.1155/2012/417267
Randomised, double blind, placebo-controlled trial of echinacea supplementation in air travellers
Abstract
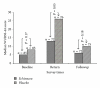
Objective. To identify whether a standardised Echinacea formulation is effective in the prevention of respiratory and other symptoms associated with long-haul flights. Methods. 175 adults participated in a randomised, double-blind placebo-controlled trial travelling back from Australia to America, Europe, or Africa for a period of 1-5 weeks on commercial flights via economy class. Participants took Echinacea (root extract, standardised to 4.4 mg alkylamides) or placebo tablets. Participants were surveyed before, immediately after travel, and at 4 weeks after travel regarding upper respiratory symptoms and travel-related quality of life. Results. Respiratory symptoms for both groups increased significantly during travel (P < 0.0005). However, the Echinacea group had borderline significantly lower respiratory symptom scores compared to placebo (P = 0.05) during travel. Conclusions. Supplementation with standardised Echinacea tablets, if taken before and during travel, may have preventive effects against the development of respiratory symptoms during travel involving long-haul flights.
Figures
References
-
- Norbäck D, Lindgren T, Wieslander G. Changes in ocular and nasal signs and symptons among air crew in relation to air humidification on intercontinental flights. Scandinavian Journal of Work, Environment and Health. 2006;32(2):138–144. - PubMed
-
- Ohrui N, Takeuchi A, Tong A, Iwata M, Nakamura A, Ohashi K. Allergic rhinitis and ear pain in flight. Annals of Allergy, Asthma and Immunology. 2005;95(4):350–353. - PubMed
-
- Powell B, Ford C. Risks of travel, benefits of a specialist consult. Cleveland Clinic Journal of Medicine. 2010;77(4):246–254. - PubMed
LinkOut - more resources
Full Text Sources