Novel Modeling Approach to Generate a Polymeric Nanofiber Scaffold for Salivary Gland Cells
- PMID: 22229076
- PMCID: PMC3252387
- DOI: 10.1115/1.4001744
Novel Modeling Approach to Generate a Polymeric Nanofiber Scaffold for Salivary Gland Cells
Abstract
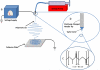
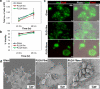
BACKGROUND: Electrospun nanofibers have been utilized in many biomedical applications as biomimetics of extracellular matrix proteins that promote self-organization of cells into 3D tissue constructs. As progress towards an artificial salivary gland tissue construct, we prepared nanofiber scaffolds using PLGA, a biodegradable and biocompatible material. METHOD OF APPROACH: We used electrospinning to prepare nanofiber scaffolds using PLGA with both DMF and HFIP as solvents. Using a design of experiment (DOE) approach, system and process parameters were optimized concurrently and their effects on the diameter of the resulting fibers were computed into a single model. A transfer function was used to reproducibly produce nanofibers of a defined diameter, which was confirmed by SEM. The mouse salivary gland epithelial cell line, SIMS, was seeded on the nanofiber scaffolds, and morphology, cell proliferation, and viability were assayed. RESULTS: Varying two or more parameters simultaneously yielded trends diverging from the linear response predicted by previous studies. Comparison of two solvents revealed that the diameter of PLGA nanofibers generated using HFIP is less sensitive to changes in the system and process parameters than are fibers generated using DMF. Inclusion of NaCl reduced morphological inconsistencies and minimized process variability. The resulting nanofiber scaffolds supported attachment, survival and cell proliferation of a mouse salivary gland epithelial cell line. In comparison with glass and flat PLGA films, the nanofibers promoted self-organization of the salivary gland cells into 3D cell clusters, or aggregates. CONCLUSIONS: These data indicate that nanofiber scaffolds promote salivary gland cell organization, and suggest that a nanofiber scaffold could provide a platform for engineering of an artificial salivary gland tissue construct. This study additionally provides a method for efficient production of nanofiber scaffolds for general application in tissue engineering.
Figures




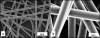
References
-
- Delaleu N, Jonsson R, Koller MM. Sjogren's syndrome. European journal of oral sciences. 2005;113(2):101–113. - PubMed
-
- Bhide SA, Miah AB, Harrington KJ, Newbold KL, Nutting CM. Radiation-induced xerostomia: pathophysiology, prevention and treatment. Clinical oncology (Royal College of Radiologists (Great Britain)) 2009;21(10):737–744. - PubMed
-
- Pedersen AM, Nauntofte B. Primary Sjogren's syndrome: oral aspects on pathogenesis, diagnostic criteria, clinical features and approaches for therapy. Expert opinion on pharmacotherapy. 2001;2(9):1415–1436. - PubMed
-
- Napenas JJ, Brennan MT, Fox PC. Diagnosis and treatment of xerostomia (dry mouth) Odontology / the Society of the Nippon Dental University. 2009;97(2):76–83. - PubMed
-
- Mese H, Matsuo R. Salivary secretion, taste and hyposalivation. Journal of oral rehabilitation. 2007;34(10):711–723. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
