Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation
- PMID: 22229260
- PMCID: PMC3329950
- DOI: 10.1089/ars.2011.4173
Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation
Abstract
Aims: Intracellular amyloid beta (Aβ) oligomers and extracellular Aβ plaques are key players in the progression of sporadic Alzheimer's disease (AD). Still, the molecular signals triggering Aβ production are largely unclear. We asked whether mitochondrion-derived reactive oxygen species (ROS) are sufficient to increase Aβ generation and thereby initiate a vicious cycle further impairing mitochondrial function.
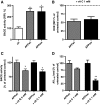
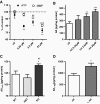
Results: Complex I and III dysfunction was induced in a cell model using the respiratory inhibitors rotenone and antimycin, resulting in mitochondrial dysfunction and enhanced ROS levels. Both treatments lead to elevated levels of Aβ. Presence of an antioxidant rescued mitochondrial function and reduced formation of Aβ, demonstrating that the observed effects depended on ROS. Conversely, cells overproducing Aβ showed impairment of mitochondrial function such as comprised mitochondrial respiration, strongly altered morphology, and reduced intracellular mobility of mitochondria. Again, the capability of these cells to generate Aβ was partly reduced by an antioxidant, indicating that Aβ formation was also ROS dependent. Moreover, mice with a genetic defect in complex I, or AD mice treated with a complex I inhibitor, showed enhanced Aβ levels in vivo.
Innovation: We show for the first time that mitochondrion-derived ROS are sufficient to trigger Aβ production in vitro and in vivo.
Conclusion: Several lines of evidence show that mitochondrion-derived ROS result in enhanced amyloidogenic amyloid precursor protein processing, and that Aβ itself leads to mitochondrial dysfunction and increased ROS levels. We propose that starting from mitochondrial dysfunction a vicious cycle is triggered that contributes to the pathogenesis of sporadic AD.
Figures






References
-
- Anandatheerthavarada HK. Devi L. Amyloid precursor protein and mitochondrial dysfunction in Alzheimer's disease. Neuroscientist. 2007;13:626–638. - PubMed
-
- Andreyev AI. Kushnareva YE. Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochem Mosc. 2005;70:200–214. - PubMed
-
- Behl C. Apoptosis and Alzheimer's disease. J Neural Transm. 2000;107:1325–1344. - PubMed
-
- Benard G. Bellance N. James D. Parrone P. Fernandez H. Letellier T. Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci. 2007;120:838–848. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

