Degradation of nuclease-stabilized RNA oligonucleotides in Mycoplasma-contaminated cell culture media
- PMID: 22229275
- PMCID: PMC3318257
- DOI: 10.1089/nat.2011.0316
Degradation of nuclease-stabilized RNA oligonucleotides in Mycoplasma-contaminated cell culture media
Abstract
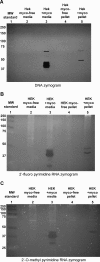
Artificial RNA reagents such as small interfering RNAs (siRNAs) and aptamers often must be chemically modified for optimal effectiveness in environments that include ribonucleases. Mycoplasmas are common bacterial contaminants of mammalian cell cultures that are known to produce ribonucleases. Here we describe the rapid degradation of nuclease-stabilized RNA oligonucleotides in a human embryonic kidney 293 (HEK) cell culture contaminated with Mycoplasma fermentans, a common species of mycoplasma. RNA with 2'-fluoro- or 2'-O-methyl- modified pyrimidines was readily degraded in conditioned media from this culture, but was stable in conditioned media from uncontaminated HEK cells. RNA completely modified with 2'-O-methyls was not degraded in the mycoplasma-contaminated media. RNA zymogram analysis of conditioned culture media and material centrifuged from the media revealed several distinct protein bands (ranging from 30 to 68 kDa) capable of degrading RNA with 2'-fluoro- or 2'-O-methyl-modified pyrimidines. Finally, the mycoplasma-associated nuclease was detected in material centrifuged from the contaminated culture supernatants in as little as 15 minutes with an RNA oligo-containing 2'-O-methyl-modified pyrimidines and labeled with a 5'-fluorescein amidite (FAM) and 3'-quencher. These results suggest that mycoplasma contamination may be a critical confounding variable for cell culture experiments involving RNA-based reagents, with particular relevance for applications involving naked RNA (e.g., aptamer-siRNA chimeras).
Figures






Similar articles
-
Inhibition of HIV type 1 reverse transcriptase assay by nucleases produced by contaminating mycoplasmas.AIDS Res Hum Retroviruses. 1994 Oct;10(10):1251-7. doi: 10.1089/aid.1994.10.1251. AIDS Res Hum Retroviruses. 1994. PMID: 7531461
-
Suppression of HIV-1 reverse transcriptase activity by culture supernatants of mycoplasmas.Microbiol Immunol. 1995;39(12):987-93. doi: 10.1111/j.1348-0421.1995.tb03302.x. Microbiol Immunol. 1995. PMID: 8789058
-
The metabolism of AIDS-associated mycoplasmas.Clin Infect Dis. 1993 Aug;17 Suppl 1:S267-71. doi: 10.1093/clinids/17.supplement_1.s267. Clin Infect Dis. 1993. PMID: 8399928 Review.
-
Animal model of Mycoplasma fermentans respiratory infection.BMC Res Notes. 2013 Jan 8;6:9. doi: 10.1186/1756-0500-6-9. BMC Res Notes. 2013. PMID: 23298636 Free PMC article.
-
Mycoplasmas as cofactors in infection due to the human immunodeficiency virus.Clin Infect Dis. 1993 Aug;17 Suppl 1:S309-15. Clin Infect Dis. 1993. PMID: 8399934 Review.
Cited by
-
Aptamers: precision tools for diagnosing and treating infectious diseases.Front Cell Infect Microbiol. 2024 Sep 25;14:1402932. doi: 10.3389/fcimb.2024.1402932. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39386170 Free PMC article. Review.
-
The effects of mycoplasma contamination upon the ability to form bioengineered 3D kidney cysts.PLoS One. 2015 Mar 20;10(3):e0120097. doi: 10.1371/journal.pone.0120097. eCollection 2015. PLoS One. 2015. PMID: 25793639 Free PMC article.
-
Challenges in the use of nanostructures as carriers of nucleic acids in clinical practice.Einstein (Sao Paulo). 2022 Feb 21;20:eRB5898. doi: 10.31744/einstein_journal/2022RB5898. eCollection 2022. Einstein (Sao Paulo). 2022. PMID: 35195162 Free PMC article. Review.
-
Method for Confirming Cytoplasmic Delivery of RNA Aptamers.Methods Mol Biol. 2016;1364:209-17. doi: 10.1007/978-1-4939-3112-5_17. Methods Mol Biol. 2016. PMID: 26472453 Free PMC article.
-
Discovery and Proof-of-Concept Study of Nuclease Activity as a Novel Biomarker for Breast Cancer Tumors.Cancers (Basel). 2021 Jan 13;13(2):276. doi: 10.3390/cancers13020276. Cancers (Basel). 2021. PMID: 33451046 Free PMC article.
References
-
- ALLERSON C.R. SIOUFI N. JARRES R. PRAKASH T.P. NAIK N. BERDEJA A. WANDERS L. GRIFFEY R.H. SWAYZE E.E. BHAT B. Fully 2′-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering RNA. J. Med. Chem. 2005;48:901–904. - PubMed
-
- BEHLKE M.A. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18:305–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

