A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients
- PMID: 22229758
- PMCID: PMC3668312
- DOI: 10.1037/a0026883
A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients
Abstract
Objective: Contingency management (CM) reduces drug use, but questions remain regarding optimal targets and magnitudes of reinforcement. We evaluated the efficacy of CM reinforcing attendance in patients who initiated treatment with cocaine-negative samples, and of higher magnitude abstinence-based CM in patients who began treatment positive.
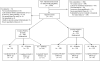
Method: Initially cocaine-negative patients (n = 333) were randomized to standard care (SC), SC + CM reinforcing submission of negative samples with $250 in prizes ($250Abs), or SC + CM reinforcing attendance ($250Att). Initially cocaine-positive patients (n = 109) were randomized to SC, $250Abs, or higher magnitude CM ($560Abs).
Results: For initially cocaine-negative patients, $250Abs and $250Att were equally efficacious to SC in enhancing longest duration of abstinence (LDA); $250Att patients submitted lower proportions of negative samples when missing samples were considered missing, but these patients also attended more study sessions, provided more samples, and submitted a higher proportion of negative samples than SC patients when expected samples were analyzed, ps < .05. In initially cocaine-positive patients, both CM conditions increased proportions of negative samples relative to SC when missing samples were excluded from analyses, but only $560Abs was efficacious in increasing LDA and proportion of negative samples when expected samples were analyzed, ps < .05. Follow-ups revealed no differences among groups, but LDA was consistently associated with abstinence during follow-up, p < .05.
Conclusions: High magnitude abstinence-based reinforcement improved all abstinence outcomes in patients who began treatment while using cocaine. For patients initiating treatment abstinent, both attendance- and abstinence-based CM resulted in improvements on some measures.
PsycINFO Database Record (c) 2012 APA, all rights reserved.
References
-
- Alessi SM, Hanson T, Wieners M, Petry NM. Low-cost contingency management in community clinics: delivering incentives partially in group therapy. Experimental and Clinical Psychopharmacology. 2007;15:293–300. - PubMed
-
- Alterman AI, Kampman K, Boardman CR, Cacciola JS, Rutherford MJ, McKay JR, et al. A cocaine-positive baseline urine predicts outpatient treatment attrition and failure to attain initial abstinence. Drug and Alcohol Dependence. 1997;46:79–85. - PubMed
-
- Alterman AI, McKay JR, Mulvaney FD, McLellan AT. Prediction of attrition from day hospital treatment in lower socioeconomic cocaine-dependent men. Drug and Alcohol Dependence. 1996;40:227–233. - PubMed
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: American Psychiatric Association; 2000. text revision (DSM-IV-TR)
-
- Bovasso GB, Alterman AI, Cacciola JS, Cook TG. Predictive validity of the Addiction Severity Index's composite scores in the assessment of 2-year outcomes in a methadone maintenance population. Psychology of Addictive Behavior. 2001;15:171–176. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DA013444/DA/NIDA NIH HHS/United States
- P30-DA023918/DA/NIDA NIH HHS/United States
- R01-DA018883/DA/NIDA NIH HHS/United States
- R01-DA024667/DA/NIDA NIH HHS/United States
- P50 DA009241/DA/NIDA NIH HHS/United States
- R01 DA021567/DA/NIDA NIH HHS/United States
- R01 DA024667/DA/NIDA NIH HHS/United States
- R01 DA018883/DA/NIDA NIH HHS/United States
- M01 RR006192/RR/NCRR NIH HHS/United States
- R01-DA021567/DA/NIDA NIH HHS/United States
- R01 DA016855/DA/NIDA NIH HHS/United States
- P60-AA03510/AA/NIAAA NIH HHS/United States
- R01-DA022739/DA/NIDA NIH HHS/United States
- P60 AA003510/AA/NIAAA NIH HHS/United States
- R01-DA016855/DA/NIDA NIH HHS/United States
- R01-DA027615/DA/NIDA NIH HHS/United States
- R01-DA13444/DA/NIDA NIH HHS/United States
- R01 DA027615/DA/NIDA NIH HHS/United States
- R01 DA022739/DA/NIDA NIH HHS/United States
- M01-RR06192/RR/NCRR NIH HHS/United States
- P30 DA023918/DA/NIDA NIH HHS/United States
- P50-DA09241/DA/NIDA NIH HHS/United States