Rett syndrome induced pluripotent stem cell-derived neurons reveal novel neurophysiological alterations
- PMID: 22230884
- PMCID: PMC3504383
- DOI: 10.1038/mp.2011.180
Rett syndrome induced pluripotent stem cell-derived neurons reveal novel neurophysiological alterations
Abstract
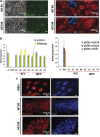
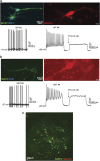
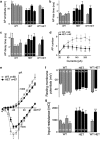
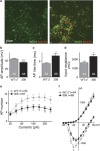
Rett syndrome (RTT) is a neurodevelopmental autism spectrum disorder caused by mutations in the methyl-CpG-binding protein 2 (MECP2) gene. Here, we describe the first characterization and neuronal differentiation of induced pluripotent stem (iPS) cells derived from Mecp2-deficient mice. Fully reprogrammed wild-type (WT) and heterozygous female iPS cells express endogenous pluripotency markers, reactivate the X-chromosome and differentiate into the three germ layers. We directed iPS cells to produce glutamatergic neurons, which generated action potentials and formed functional excitatory synapses. iPS cell-derived neurons from heterozygous Mecp2(308) mice showed defects in the generation of evoked action potentials and glutamatergic synaptic transmission, as previously reported in brain slices. Further, we examined electrophysiology features not yet studied with the RTT iPS cell system and discovered that MeCP2-deficient neurons fired fewer action potentials, and displayed decreased action potential amplitude, diminished peak inward currents and higher input resistance relative to WT iPS-derived neurons. Deficiencies in action potential firing and inward currents suggest that disturbed Na(+) channel function may contribute to the dysfunctional RTT neuronal network. These phenotypes were additionally confirmed in neurons derived from independent WT and hemizygous mutant iPS cell lines, indicating that these reproducible deficits are attributable to MeCP2 deficiency. Taken together, these results demonstrate that neuronally differentiated MeCP2-deficient iPS cells recapitulate deficits observed previously in primary neurons, and these identified phenotypes further illustrate the requirement of MeCP2 in neuronal development and/or in the maintenance of normal function. By validating the use of iPS cells to delineate mechanisms underlying RTT pathogenesis, we identify deficiencies that can be targeted for in vitro translational screens.
Figures

References
-
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
-
- Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: report of 35 cases. Ann Neurol. 1983;14:471–479. - PubMed
-
- Jung BP, Jugloff DG, Zhang G, Logan R, Brown S, Eubanks JH. The expression of methyl CpG binding factor MeCP2 correlates with cellular differentiation in the developing rat brain and in cultured cells. J Neurobiol. 2003;55:86–96. - PubMed
-
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

