Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes
- PMID: 22230951
- PMCID: PMC3313462
- DOI: 10.1038/nrmicro2714
Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes
Abstract
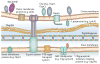
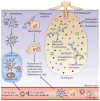
In little more than 30 years, Lyme disease, which is caused by the spirochaete Borrelia burgdorferi, has risen from relative obscurity to become a global public health problem and a prototype of an emerging infection. During this period, there has been an extraordinary accumulation of knowledge on the phylogenetic diversity, molecular biology, genetics and host interactions of B. burgdorferi. In this Review, we integrate this large body of information into a cohesive picture of the molecular and cellular events that transpire as Lyme disease spirochaetes transit between their arthropod and vertebrate hosts during the enzootic cycle.
Figures



References
-
- Benach JL, Garcia Monco JC. In: Borrelia: Molecular Biology, Host Interaction, and Pathogenesis. Samuels DS, Radolf JD, editors. Caister Academic; Norfolk, UK: 2010. pp. 7–26.
-
- Steere AC, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 1977;20:7–17. This article describes the original epidemiological study of the outbreak of arthritis in and around Lyme, Connecticut. - PubMed
-
- Burgdorfer W, et al. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. This classic paper provides the first convincing evidence that Lyme disease is a tick-borne illness. - PubMed
-
- Steere AC, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308:733–740. - PubMed
-
- Benach JL, et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308:740–742. This and reference 4 are the first two reports to describe the isolation of Lyme disease spirochaetes from patients. - PubMed
Publication types
MeSH terms
Grants and funding
- AI044254/AI/NIAID NIH HHS/United States
- R01 AI071107/AI/NIAID NIH HHS/United States
- R03 AI085248/AI/NIAID NIH HHS/United States
- R56 AI080646/AI/NIAID NIH HHS/United States
- AI068799/AI/NIAID NIH HHS/United States
- AI085248/AI/NIAID NIH HHS/United States
- AI080646/AI/NIAID NIH HHS/United States
- R56 AI044254/AI/NIAID NIH HHS/United States
- AI082436/AI/NIAID NIH HHS/United States
- R21 AI082436/AI/NIAID NIH HHS/United States
- AI029735/AI/NIAID NIH HHS/United States
- AI071107/AI/NIAID NIH HHS/United States
- R01 AI044254/AI/NIAID NIH HHS/United States
- R41 AI078631/AI/NIAID NIH HHS/United States
- R56 AI029735/AI/NIAID NIH HHS/United States
- R01 AI029735/AI/NIAID NIH HHS/United States
- AI029735‑20S1/AI/NIAID NIH HHS/United States
- R01 AI068799/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

