X-ray structures of LeuT in substrate-free outward-open and apo inward-open states
- PMID: 22230955
- PMCID: PMC3306218
- DOI: 10.1038/nature10737
X-ray structures of LeuT in substrate-free outward-open and apo inward-open states
Abstract
Neurotransmitter sodium symporters are integral membrane proteins that remove chemical transmitters from the synapse and terminate neurotransmission mediated by serotonin, dopamine, noradrenaline, glycine and GABA (γ-aminobutyric acid). Crystal structures of the bacterial homologue, LeuT, in substrate-bound outward-occluded and competitive inhibitor-bound outward-facing states have advanced our mechanistic understanding of neurotransmitter sodium symporters but have left fundamental questions unanswered. Here we report crystal structures of LeuT mutants in complexes with conformation-specific antibody fragments in the outward-open and inward-open states. In the absence of substrate but in the presence of sodium the transporter is outward-open, illustrating how the binding of substrate closes the extracellular gate through local conformational changes: hinge-bending movements of the extracellular halves of transmembrane domains 1, 2 and 6, together with translation of extracellular loop 4. The inward-open conformation, by contrast, involves large-scale conformational changes, including a reorientation of transmembrane domains 1, 2, 5, 6 and 7, a marked hinge bending of transmembrane domain 1a and occlusion of the extracellular vestibule by extracellular loop 4. These changes close the extracellular gate, open an intracellular vestibule, and largely disrupt the two sodium sites, thus providing a mechanism by which ions and substrate are released to the cytoplasm. The new structures establish a structural framework for the mechanism of neurotransmitter sodium symporters and their modulation by therapeutic and illicit substances.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

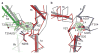
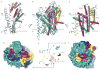


References
-
- Hertting G, Axelrod J. Fate of tritiated noradrenaline at the sympathetic nerve-endings. Nature. 1961;192:172–173. - PubMed
-
- Nelson N. The family of Na+/Cl- neurotransmitter transporters. J Neurochem. 1998;71:1785–1803. - PubMed
-
- Hahn MK, Blakely RD. Monoamine transporter gene structure and polymorphisms in relation to psychiatric and other complex disorders. Pharmacogenomics J. 2002;2:217–235. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

