Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer
- PMID: 22231041
- PMCID: PMC3295555
- DOI: 10.1200/JCO.2011.34.6890
Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer
Abstract
Purpose: Chemotherapy-induced amenorrhea is a serious concern for women undergoing cancer therapy. This prospective randomized trial evaluated the use of gonadotropin-releasing hormone (GnRH) analog triptorelin to preserve ovarian function in women treated with chemotherapy for early-stage breast cancer.
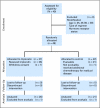
Patients and methods: Premenopausal women age 44 years or younger were randomly assigned to receive either triptorelin or no triptorelin during (neo)adjuvant chemotherapy and were further stratified by age (< 35, 35 to 39, > 39 years), estrogen receptor status, and chemotherapy regimen. Objectives included the resumption of menses and serial monitoring of follicle-stimulating hormone (FSH) and inhibin A and B levels.
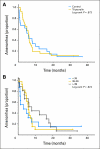
Results: Targeted for 124 patients with a planned 5-year follow-up, the trial was stopped for futility after 49 patients were enrolled (median age, 39 years; range, 21 to 43 years); 47 patients were treated according to assigned groups with four cycles of adriamycin plus cyclophosphamide alone or followed by four cycles of paclitaxel or six cycles of fluorouracil, epirubicin, and cyclophosphamide. Menstruation resumed in 19 (90%) of 21 patients in the control group and in 23 (88%) of 26 in the triptorelin group (P= .36). Menses returned after a median of 5.8 months (range, 1 to 19 months) after completion of chemotherapy in the triptorelin versus 5.0 months (range, 0 to 28 months) in the control arm (P= .58). Two patients (age 26 and 35 years at random assignment) in the control group had spontaneous pregnancies with term deliveries. FSH and inhibin B levels correlated with menstrual status.
Conclusion: When stratified for age, estrogen receptor status, and treatment regimen, amenorrhea rates on triptorelin were comparable to those seen in the control group.
Figures
Comment in
-
Ovarian suppression for prevention of premature menopause and infertility: empty promise or effective therapy?J Clin Oncol. 2012 Feb 10;30(5):479-81. doi: 10.1200/JCO.2011.37.9883. Epub 2012 Jan 9. J Clin Oncol. 2012. PMID: 22231046 No abstract available.
-
Gonadotropin-releasing hormone agonists for the preservation of ovarian function during chemotherapy.J Clin Oncol. 2012 Sep 10;30(26):3310-1; author reply 3312-3. doi: 10.1200/JCO.2012.42.2436. Epub 2012 May 29. J Clin Oncol. 2012. PMID: 22649128 No abstract available.
-
Gonadotropin-releasing hormone agonist for preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer.J Clin Oncol. 2012 Sep 10;30(26):3310; author reply 3312-3. doi: 10.1200/JCO.2012.41.8467. Epub 2012 May 29. J Clin Oncol. 2012. PMID: 22649145 No abstract available.
-
Potential late effect of gonadotropin-releasing hormone agonists in combination with chemotherapy for early breast cancer.J Clin Oncol. 2012 Sep 10;30(26):3311-2. doi: 10.1200/JCO.2012.42.6197. Epub 2012 May 29. J Clin Oncol. 2012. PMID: 22649148 No abstract available.
References
-
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344:1997–2008. - PubMed
-
- Goldhirsch A, Gelber RD, Castiglione M. The magnitude of endocrine effects of adjuvant chemotherapy for premenopausal breast cancer patients: The International Breast Cancer Study Group. Ann Oncol. 1990;1:183–188. - PubMed
-
- Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. - PubMed
-
- Fornier MN, Modi S, Panageas KS, et al. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer. 2005;104:1575–1579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical