Bone scan index: a quantitative treatment response biomarker for castration-resistant metastatic prostate cancer
- PMID: 22231045
- PMCID: PMC3295554
- DOI: 10.1200/JCO.2011.36.5791
Bone scan index: a quantitative treatment response biomarker for castration-resistant metastatic prostate cancer
Abstract
Purpose: There is currently no imaging biomarker for metastatic prostate cancer. The bone scan index (BSI) is a promising candidate, being a reproducible, quantitative expression of tumor burden seen on bone scintigraphy. Prior studies have shown the prognostic value of a baseline BSI. This study tested whether treatment-related changes in BSI are prognostic for survival and compared BSI to prostate-specific antigen (PSA) as an outcome measure.
Patients and methods: We retrospectively examined serial bone scans from patients with castration-resistant metastatic prostate cancer (CRMPC) enrolled in four clinical trials. We calculated BSI at baseline and at 3 and 6 months on treatment and performed univariate and bivariate analyses of PSA, BSI, and survival.
Results: Eighty-eight patients were scanned, 81 of whom have died. In the univariate analysis, the log percent change in BSI from baseline to 3 and 6 months on treatment prognosticated for survival (hazard ratio [HR], 2.44; P = .0089 and HR, 2.54; P < .001, respectively). A doubling in BSI resulted in a 1.9-fold increase in risk of death. Log percent change in PSA at 6 months on treatment was also associated with survival (HR, 1.298; P = .013). In the bivariate analysis, change in BSI while adjusting for PSA was prognostic at 3 and 6 months on treatment (HR, 2.368; P = .012 and HR, 2.226; P = .002, respectively), but while adjusting for BSI, PSA was not prognostic.
Conclusion: These data furnish early evidence that on-treatment changes in BSI are a response indicator and support further exploration of bone scintigraphy as an imaging biomarker in CRMPC.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
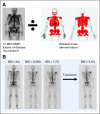

Comment in
-
Prostate cancer: bone scan index--a new imaging biomarker?Nat Rev Urol. 2012 Jan 24;9(2):61. doi: 10.1038/nrurol.2012.6. Nat Rev Urol. 2012. PMID: 22270137 No abstract available.
References
-
- Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer. 1988;61:195–202. - PubMed
-
- Amico S, Liehn JC, Desoize B, et al. Comparison of phosphatase isoenzymes PAP and PSA with bone scan in patients with prostate carcinoma. Clin Nucl Med. 1991;16:643–648. - PubMed
-
- Chybowski FM, Keller JJ, Bergstralh EJ, et al. Predicting radionuclide bone scan findings in patients with newly diagnosed, untreated prostate cancer: Prostate specific antigen is superior to all other clinical parameters. J Urol. 1991;145:313–318. - PubMed
-
- Imbriaco M, Larson SM, Yeung HW, et al. A new parameter for measuring metastatic bone involvement by prostate cancer: The Bone Scan Index. Clin Cancer Res. 1998;4:1765–1772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

