A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP
- PMID: 22231303
- PMCID: PMC3260865
- DOI: 10.1084/jem.20110896
A novel primary human immunodeficiency due to deficiency in the WASP-interacting protein WIP
Abstract
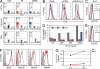
A female offspring of consanguineous parents, showed features of Wiskott-Aldrich syndrome (WAS), including recurrent infections, eczema, thrombocytopenia, defective T cell proliferation and chemotaxis, and impaired natural killer cell function. Cells from this patient had undetectable WAS protein (WASP), but normal WAS sequence and messenger RNA levels. WASP interacting protein (WIP), which stabilizes WASP, was also undetectable. A homozygous c.1301C>G stop codon mutation was found in the WIPF1 gene, which encodes WIP. Introduction of WIP into the patient's T cells restored WASP expression. These findings indicate that WIP deficiency should be suspected in patients with features of WAS in whom WAS sequence and mRNA levels are normal.
Figures

References
-
- Castriconi R., Dondero A., Cilli M., Ognio E., Pezzolo A., De Giovanni B., Gambini C., Pistoia V., Moretta L., Moretta A., Corrias M.V. 2007. Human NK cell infusions prolong survival of metastatic human neuroblastoma-bearing NOD/scid mice. Cancer Immunol. Immunother. 56:1733–1742 10.1007/s00262-007-0317-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

