AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation
- PMID: 22231556
- PMCID: PMC3274651
- DOI: 10.1038/nm.2598
AICAR prevents heat-induced sudden death in RyR1 mutant mice independent of AMPK activation
Abstract
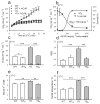
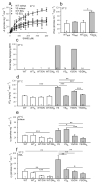
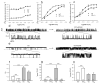
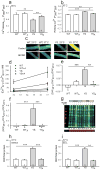
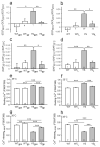
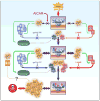
Mice with a knock-in mutation (Y524S) in the type I ryanodine receptor (Ryr1), a mutation analogous to the Y522S mutation that is associated with malignant hyperthermia in humans, die when exposed to short periods of temperature elevation (≥37 °C). We show here that treatment with 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) prevents this heat-induced sudden death in this mouse model. The protection by AICAR is independent of AMP-activated protein kinase (AMPK) activation and results from a newly identified action of the compound on mutant Ryr1 to reduce Ca(2+) leak from the sarcoplasmic reticulum to the sarcoplasm. AICAR thus prevents Ca(2+)-dependent increases in the amount of both reactive oxygen species (ROS) and reactive nitrogen species (RNS) that act to further increase resting Ca(2+) concentrations. If unchecked, the temperature-driven increases in resting Ca(2+) concentrations and the amounts of ROS and RNS create an amplifying cycle that ultimately triggers sustained muscle contractions, rhabdomyolysis and death. Although antioxidants are effective in reducing this cycle in vitro, only AICAR prevents heat-induced death in vivo. Our findings suggest that AICAR is probably effective in prophylactic treatment of humans with enhanced susceptibility to exercise- and/or heat-induced sudden death associated with RYR1 mutations.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
-
- Bouchama A, Knochel JP. Heat Stroke. N Engl J Med. 2002;346(25):1978–88. - PubMed
-
- Hopkins PM, Ellis FR, Halsall PJ. Evidence for related myopathies in exertional heat stroke and malignant hyperthermia. The Lancet. 1991;338:1491–92. - PubMed
-
- Jurkat-Rott K, McCarthy T, Lehmann-Horn F. Genetics and Pathogenesis of Malignant Hyperthermia. Muscle Nerve. 2000;23(1):4–17. - PubMed
-
- Wappler F, Fiege M, Steinfath M, Agarwal K, Scholz J, Singh S, Matschke J, Schulte Am Esch J. Evidence for Susceptibility to Malignant Hyperthermia in Patients with Exercise-Induced Rhabdomyolysis. Anesthesiology. 2001;94(1):95–100. - PubMed
-
- Davis M, Brown R, Dickson A, Horton H, James D, Laing N, Marston R, Norgate M, Perlman D, Pollock N, Stonwell K. Malignant Hyperthermia Associated with Exercise-Induced Rhabdomyolysis or Congenital Abnormalities and a Novel RYR1 Mutation in New Zealand and Australian Pedigrees. Br J Anaesth. 2002;88(4):508–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

