Effect of prostaglandin I2 analogs on cytokine expression in human myeloid dendritic cells via epigenetic regulation
- PMID: 22231731
- PMCID: PMC3356435
- DOI: 10.2119/molmed.2011.00193
Effect of prostaglandin I2 analogs on cytokine expression in human myeloid dendritic cells via epigenetic regulation
Abstract
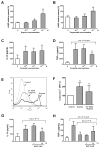
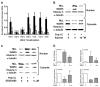
Prostaglandin I(2) (PGI(2)) analog is regarded as a potential candidate for treating asthma. Human myeloid dendritic cells (mDCs) play a critical role in the pathogenesis of asthma. However, the effects of PGI(2) analog on human mDCs are unknown. In the present study, circulating mDCs were isolated from six healthy subjects. The effects of PGI(2) analogs iloprost and treprostinil on cytokine production, maturation and T-cell stimulatory function of human mDCs were investigated. Tumor necrosis factor (TNF)-α and interleukin (IL)-10 were measured by enzyme-linked immunosorbent assay. The expression of costimulatory molecules was investigated by flow cytometry. T-cell stimulatory function was investigated by measuring interferon (IFN)-γ, IL-13 and IL-10 production by T cells cocultured with iloprost-treated mDCs. Intracellular signaling was investigated by Western blot and chromatin immunoprecipitation. We found that iloprost and treprostinil induced IL-10, but suppressed TNF-α production in polyinosinic-polycytidylic acid (poly I:C)-stimulated mDCs. This effect was reversed by the I-prostanoid (IP), E-prostanoid (EP) receptor antagonists or intracellular free calcium (Ca(2+)) chelator. Forskolin, an adenyl cyclase activator, conferred a similar effect. Iloprost and treprostinil increased intracellular adenosine 3',5'-cyclic monophosphate (cAMP) levels, and iloprost also increased intracellular Ca(2+). Iloprost suppressed poly I:C-induced mitogen-activated protein kinase (MAPK) phospho-p38 and phospho-activating transcription factor (ATF)2 expression. Iloprost downregulated poly I:C-induced histone H3K4 trimethylation in the TNFA gene promoter region via suppressing translocation of histone 3 lysine 4 (H3K4)-specific methyltransferases MLL (mixed lineage leukemia) and WDR5 (WD repeat domain 5). Iloprost-treated mDCs inhibited IL-13, IFN-γ and IL-10 production by T cells. In conclusion, PGI(2) analogs enhance IL-10 and suppress TNF-α expression through the IP/EP2/EP4 receptors-cAMP and EP1 receptor-Ca(2+) pathway. Iloprost suppressed TNF-α expression via the MAPK-p38-ATF2 pathway and epigenetic regulation by downregulation of histone H3K4 trimethylation.
Figures
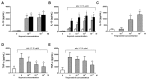
 , 24 h; ■, 48 h.
, 24 h; ■, 48 h.

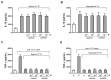

 , iloprost + poly I:C.
, iloprost + poly I:C.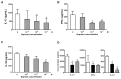
 , iloprost (10−7 mol/L) + anti–IL-10.
, iloprost (10−7 mol/L) + anti–IL-10.Similar articles
-
Prostaglandin I(2) analogues enhance growth-related oncogene-alpha expression in human monocyte-derived dendritic cells.Inflammation. 2010 Oct;33(5):334-43. doi: 10.1007/s10753-010-9190-7. Inflammation. 2010. PMID: 20195728
-
Prostaglandin I2 analogs suppress tumor necrosis factor α production and the maturation of human monocyte-derived dendritic cells.J Investig Med. 2011 Oct;59(7):1109-15. doi: 10.2310/JIM.0b013e3182281f62. J Investig Med. 2011. PMID: 21716128
-
Effects of PGI2 analogues on Th1- and Th2-related chemokines in monocytes via epigenetic regulation.J Mol Med (Berl). 2011 Jan;89(1):29-41. doi: 10.1007/s00109-010-0694-2. Epub 2010 Nov 18. J Mol Med (Berl). 2011. PMID: 21085923
-
Cysteinyl leukotriene receptor antagonist epigenetically modulates cytokine expression and maturation of human myeloid dendritic cells.Pulm Pharmacol Ther. 2016 Aug;39:28-37. doi: 10.1016/j.pupt.2016.06.001. Epub 2016 Jun 14. Pulm Pharmacol Ther. 2016. PMID: 27312202
-
Regulation of cytokine expression in human plasmacytoid dendritic cells by prostaglandin I2 analogues.Eur Respir J. 2009 Feb;33(2):405-10. doi: 10.1183/09031936.00070008. Eur Respir J. 2009. PMID: 19181914
Cited by
-
H3K4 trimethylation regulates cancer immunity: a promising therapeutic target in combination with immunotherapy.J Immunother Cancer. 2023 Aug;11(8):e005693. doi: 10.1136/jitc-2022-005693. J Immunother Cancer. 2023. PMID: 37553181 Free PMC article. Review.
-
Suppressive Effects of 4-(Phenylsulfanyl) Butan-2-One on CCL-1 Production via Histone Acetylation in Monocytes.Curr Issues Mol Biol. 2022 Oct 3;44(10):4616-4625. doi: 10.3390/cimb44100315. Curr Issues Mol Biol. 2022. PMID: 36286030 Free PMC article.
-
Histone modifications and their role in epigenetics of atopy and allergic diseases.Allergy Asthma Clin Immunol. 2018 May 23;14:39. doi: 10.1186/s13223-018-0259-4. eCollection 2018. Allergy Asthma Clin Immunol. 2018. PMID: 29796022 Free PMC article. Review.
-
Immunological Profiles in Parry-Romberg Syndrome: A Case-Control Study.J Clin Med. 2024 Feb 21;13(5):1219. doi: 10.3390/jcm13051219. J Clin Med. 2024. PMID: 38592689 Free PMC article.
-
Epigenetic regulation in allergic diseases and related studies.Asia Pac Allergy. 2014 Jan;4(1):14-8. doi: 10.5415/apallergy.2014.4.1.14. Epub 2014 Jan 31. Asia Pac Allergy. 2014. PMID: 24527405 Free PMC article. Review.
References
-
- Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50:515–96. - PubMed
-
- Broide DH, et al. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–67. - PubMed
-
- Berry MA, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354:697–708. - PubMed
-
- Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

