Effect of nuclear factor κB inhibition on serotype 9 adeno-associated viral (AAV9) minidystrophin gene transfer to the mdx mouse
- PMID: 22231732
- PMCID: PMC3356420
- DOI: 10.2119/molmed.2011.00404
Effect of nuclear factor κB inhibition on serotype 9 adeno-associated viral (AAV9) minidystrophin gene transfer to the mdx mouse
Abstract
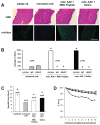
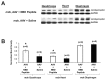
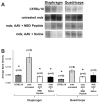
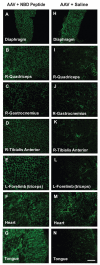
Gene therapy studies for Duchenne muscular dystrophy (DMD) have focused on viral vector-mediated gene transfer to provide therapeutic protein expression or treatment with drugs to limit dystrophic changes in muscle. The pathological activation of the nuclear factor (NF)-κB signaling pathway has emerged as an important cause of dystrophic muscle changes in muscular dystrophy. Furthermore, activation of NF-κB may inhibit gene transfer by promoting inflammation in response to the transgene or vector. Therefore, we hypothesized that inhibition of pathological NF-κB activation in muscle would complement the therapeutic benefits of dystrophin gene transfer in the mdx mouse model of DMD. Systemic gene transfer using serotype 9 adeno-associated viral (AAV9) vectors is promising for treatment of preclinical models of DMD because of vector tropism to cardiac and skeletal muscle. In quadriceps of C57BL/10ScSn-Dmd(mdx)/J (mdx) mice, the addition of octalysine (8K)-NF-κB essential modulator (NEMO)-binding domain (8K-NBD) peptide treatment to AAV9 minidystrophin gene delivery resulted in increased levels of recombinant dystrophin expression suggesting that 8K-NBD treatment promoted an environment in muscle tissue conducive to higher levels of expression. Indices of necrosis and regeneration were diminished with AAV9 gene delivery alone and to a greater degree with the addition of 8K-NBD treatment. In diaphragm muscle, high-level transgene expression was achieved with AAV9 minidystoophin gene delivery alone; therefore, improvements in histological and physiological indices were comparable in the two treatment groups. The data support benefit from 8K-NBD treatment to complement gene transfer therapy for DMD in muscle tissue that receives incomplete levels of transduction by gene transfer, which may be highly significant for clinical applications of muscle gene delivery.
Figures
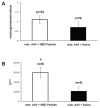
 , untreated mdx; —□—, mdx, AAV + NBD peptide; —●—, mdx, AAV + saline.
, untreated mdx; —□—, mdx, AAV + NBD peptide; —●—, mdx, AAV + saline.

Similar articles
-
Systemic delivery of NEMO binding domain/IKKγ inhibitory peptide to young mdx mice improves dystrophic skeletal muscle histopathology.Neurobiol Dis. 2011 Sep;43(3):598-608. doi: 10.1016/j.nbd.2011.05.008. Epub 2011 May 23. Neurobiol Dis. 2011. PMID: 21624467 Free PMC article.
-
D-Amino Acid Substitution of Peptide-Mediated NF-κB Suppression in mdx Mice Preserves Therapeutic Benefit in Skeletal Muscle, but Causes Kidney Toxicity.Mol Med. 2015 May 22;21(1):442-52. doi: 10.2119/molmed.2013.00141. Mol Med. 2015. PMID: 26018805 Free PMC article.
-
Inhibition of the IKK/NF-κB pathway by AAV gene transfer improves muscle regeneration in older mdx mice.Gene Ther. 2010 Dec;17(12):1476-83. doi: 10.1038/gt.2010.110. Epub 2010 Aug 19. Gene Ther. 2010. PMID: 20720575 Free PMC article.
-
Recombinant adeno-associated viral (rAAV) vectors as therapeutic tools for Duchenne muscular dystrophy (DMD).Gene Ther. 2004 Oct;11 Suppl 1:S109-21. doi: 10.1038/sj.gt.3302379. Gene Ther. 2004. PMID: 15454965 Review.
-
Contribution of oxidative stress to pathology in diaphragm and limb muscles with Duchenne muscular dystrophy.J Muscle Res Cell Motil. 2013 Feb;34(1):1-13. doi: 10.1007/s10974-012-9330-9. Epub 2012 Oct 28. J Muscle Res Cell Motil. 2013. PMID: 23104273 Review.
Cited by
-
Recent advances in Duchenne muscular dystrophy.Degener Neurol Neuromuscul Dis. 2012 Oct 11;2:141-164. doi: 10.2147/DNND.S26637. eCollection 2012. Degener Neurol Neuromuscul Dis. 2012. PMID: 30890885 Free PMC article. Review.
-
The Interplay of Mitophagy and Inflammation in Duchenne Muscular Dystrophy.Life (Basel). 2021 Jul 4;11(7):648. doi: 10.3390/life11070648. Life (Basel). 2021. PMID: 34357020 Free PMC article. Review.
-
Suppression of Activated FOXO Transcription Factors in the Heart Prolongs Survival in a Mouse Model of Laminopathies.Circ Res. 2018 Mar 2;122(5):678-692. doi: 10.1161/CIRCRESAHA.117.312052. Epub 2018 Jan 9. Circ Res. 2018. PMID: 29317431 Free PMC article.
-
Effect of IL-1β, TNF-α and IGF-1 on trans-endothelial passage of synthetic vectors through an in vitro vascular endothelial barrier of striated muscle.Gene Ther. 2017 Jul;24(7):416-424. doi: 10.1038/gt.2017.40. Epub 2017 May 15. Gene Ther. 2017. PMID: 28504656
References
-
- Emery AE. Population frequencies of inherited neuromuscular diseases: a world survey. Neuromuscul Disord. 1991;1:19–29. - PubMed
-
- Emery AEH, Muntoni F. Duchenne Muscular Dystrophy. 3rd edition. Oxford, New York: Oxford University Press; 2003. p. 270.
-
- Ervasti JM, Campbell KP. Dystrophin- associated glycoproteins: their possible roles in the pathogenesis of Duchenne muscular dystrophy. Mol Cell Biol Hum Dis Ser. 1993;3:139–66. - PubMed
-
- Hoffman E, Brown R, Kunkel L. Dystophin: the protein product of the Duchenne Muscular Dystrophy locus. Cell. 1987;51:919–28. - PubMed
-
- Zubrzycka-Gaarn EE, et al. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988;333:466–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

