Estrogen metabolism and risk of breast cancer in postmenopausal women
- PMID: 22232133
- PMCID: PMC3283536
- DOI: 10.1093/jnci/djr531
Estrogen metabolism and risk of breast cancer in postmenopausal women
Abstract
Background: Estrogens are recognized causal factors in breast cancer. Interindividual variation in estrogen metabolism may also influence the risk of breast cancer and could provide clues to mechanisms of breast carcinogenesis. Long-standing hypotheses about how estrogen metabolism might influence breast cancer have not been adequately evaluated in epidemiological studies because of the lack of accurate, reproducible, and high-throughput assays for estrogen metabolites.
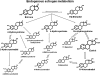
Methods: We conducted a prospective case-control study nested within the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO). Participants included 277 women who developed invasive breast cancer (case subjects) and 423 matched control subjects; at PLCO baseline, all subjects were aged 55-74 years, postmenopausal and not using hormone therapy, and provided a blood sample. Liquid chromatography-tandem mass spectrometry was used to measure serum concentrations of 15 estrogens and estrogen metabolites, in unconjugated and conjugated forms, including the parent estrogens, estrone and estradiol, and estrogen metabolites in pathways defined by irreversible hydroxylation at the C-2, C-4, or C-16 positions of the steroid ring. We calculated hazard ratios (HRs) approximating risk in highest vs lowest deciles of individual estrogens and estrogen metabolites, estrogens and estrogen metabolites grouped by metabolic pathways, and metabolic pathway ratios using multivariable Cox proportional hazards models. All statistical tests were two-sided.
Results: Nearly all estrogens, estrogen metabolites, and metabolic pathway groups were associated with an increased risk of breast cancer; the serum concentration of unconjugated estradiol was strongly associated with the risk of breast cancer (HR = 2.07, 95% confidence interval [CI] = 1.19 to 3.62). No estrogen, estrogen metabolite, or metabolic pathway group remained statistically significantly associated with the risk of breast cancer after adjusting for unconjugated estradiol. The ratio of the 2-hydroxylation pathway to parent estrogens (HR = 0.66, 95% CI = 0.51 to 0.87) and the ratio of 4-hydroxylation pathway catechols to 4-hydroxylation pathway methylated catechols (HR = 1.34, 95% CI = 1.04 to 1.72) were statistically significantly associated with the risk of breast cancer and remained so after adjustment for unconjugated estradiol.
Conclusions: More extensive 2-hydroxylation of parent estrogens is associated with lower risk, and less extensive methylation of potentially genotoxic 4-hydroxylation pathway catechols is associated with higher risk of postmenopausal breast cancer.
Figures

References
-
- Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. - PubMed
-
- Adlercreutz H, Martin F. Biliary excretion and intestinal metabolism of progesterone and estrogens in man. J Steroid Biochem. 1980;13(2):231–244. - PubMed
-
- Zhu BT, Han GZ, Shim JY, Wen Y, Jiang XR. Quantitative structure-activity relationship of various endogenous estrogen metabolites for human estrogen receptor alpha and beta subtypes: Insights into the structural determinants favoring a differential subtype binding. Endocrinology. 2006;147(9):4132–4150. - PubMed
-
- Stack DE, Byun J, Gross ML, Rogan EG, Cavalieri EL. Molecular characteristics of catechol estrogen quinones in reactions with deoxyribonucleosides. Chem Res Toxicol. 1996;9(5):851–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

