The CXCR4/CXCR7/SDF-1 pathway contributes to the pathogenesis of Shiga toxin-associated hemolytic uremic syndrome in humans and mice
- PMID: 22232208
- PMCID: PMC3266777
- DOI: 10.1172/JCI57313
The CXCR4/CXCR7/SDF-1 pathway contributes to the pathogenesis of Shiga toxin-associated hemolytic uremic syndrome in humans and mice
Abstract
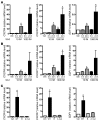
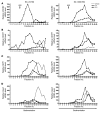
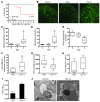
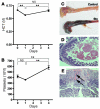
Hemolytic uremic syndrome (HUS) is a potentially life-threatening condition. It often occurs after gastrointestinal infection with E. coli O157:H7, which produces Shiga toxins (Stx) that cause hemolytic anemia, thrombocytopenia, and renal injury. Stx-mediated changes in endothelial phenotype have been linked to the pathogenesis of HUS. Here we report our studies investigating Stx-induced changes in gene expression and their contribution to the pathogenesis of HUS. Stx function by inactivating host ribosomes but can also alter gene expression at concentrations that minimally affect global protein synthesis. Gene expression profiling of human microvascular endothelium treated with Stx implicated a role for activation of CXCR4 and CXCR7 by their shared cognate chemokine ligand (stromal cell-derived factor-1 [SDF-1]) in Stx-mediated pathophysiology. The changes in gene expression required a catalytically active Stx A subunit and were mediated by enhanced transcription and mRNA stability. Stx also enhanced the association of CXCR4, CXCR7, and SDF1 mRNAs with ribosomes. In a mouse model of Stx-mediated pathology, we noted changes in plasma and tissue content of CXCR4, CXCR7, and SDF-1 after Stx exposure. Furthermore, inhibition of the CXCR4/SDF-1 interaction decreased endothelial activation and organ injury and improved animal survival. Finally, in children infected with E. coli O157:H7, plasma SDF-1 levels were elevated in individuals who progressed to HUS. Collectively, these data implicate the CXCR4/CXCR7/SDF-1 pathway in Stx-mediated pathogenesis and suggest novel therapeutic strategies for prevention and/or treatment of complications associated with E. coli O157:H7 infection.
Figures





Similar articles
-
Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells.J Exp Med. 2008 Feb 18;205(2):479-90. doi: 10.1084/jem.20071903. Epub 2008 Feb 11. J Exp Med. 2008. PMID: 18268039 Free PMC article.
-
Expression and function of the SDF-1 chemokine receptors CXCR4 and CXCR7 during mouse limb muscle development and regeneration.Exp Cell Res. 2012 Oct 15;318(17):2178-90. doi: 10.1016/j.yexcr.2012.06.020. Epub 2012 Jul 3. Exp Cell Res. 2012. PMID: 22766125
-
Experimental In Vivo Models of Bacterial Shiga Toxin-Associated Hemolytic Uremic Syndrome.J Microbiol Biotechnol. 2018 Sep 28;28(9):1413-1425. doi: 10.4014/jmb.1803.03012. J Microbiol Biotechnol. 2018. PMID: 29926707 Review.
-
Distinct renal pathology and a chemotactic phenotype after enterohemorrhagic Escherichia coli shiga toxins in non-human primate models of hemolytic uremic syndrome.Am J Pathol. 2013 Apr;182(4):1227-38. doi: 10.1016/j.ajpath.2012.12.026. Epub 2013 Feb 10. Am J Pathol. 2013. PMID: 23402998 Free PMC article.
-
New aspects in the pathogenesis of enteropathic hemolytic uremic syndrome.Semin Thromb Hemost. 2006 Mar;32(2):105-12. doi: 10.1055/s-2006-939766. Semin Thromb Hemost. 2006. PMID: 16575685 Review.
Cited by
-
Pathogenic role of inflammatory response during Shiga toxin-associated hemolytic uremic syndrome (HUS).Pediatr Nephrol. 2018 Nov;33(11):2057-2071. doi: 10.1007/s00467-017-3876-0. Epub 2018 Jan 25. Pediatr Nephrol. 2018. PMID: 29372302 Review.
-
Clinical and Laboratory Predictors of Shiga Toxin-Producing Escherichia coli Infection in Children With Bloody Diarrhea.J Pediatric Infect Dis Soc. 2018 Aug 17;7(3):e116-e122. doi: 10.1093/jpids/piy025. J Pediatric Infect Dis Soc. 2018. PMID: 29617871 Free PMC article.
-
Endothelium structure and function in kidney health and disease.Nat Rev Nephrol. 2019 Feb;15(2):87-108. doi: 10.1038/s41581-018-0098-z. Nat Rev Nephrol. 2019. PMID: 30607032 Review.
-
Renal and neurological involvement in typical Shiga toxin-associated HUS.Nat Rev Nephrol. 2012 Nov;8(11):658-69. doi: 10.1038/nrneph.2012.196. Epub 2012 Sep 18. Nat Rev Nephrol. 2012. PMID: 22986362 Review.
-
Shiga Toxin-Associated Hemolytic Uremic Syndrome: Specificities of Adult Patients and Implications for Critical Care Management.Toxins (Basel). 2021 Apr 26;13(5):306. doi: 10.3390/toxins13050306. Toxins (Basel). 2021. PMID: 33925836 Free PMC article. Review.
References
-
- Bielaszewska M, et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect Dis. 2011;11(9):671–676. - PubMed

