Hydrolysis spectrum extension of CMY-2-like β-lactamases resulting from structural alteration in the Y-X-N loop
- PMID: 22232281
- PMCID: PMC3294937
- DOI: 10.1128/AAC.05630-11
Hydrolysis spectrum extension of CMY-2-like β-lactamases resulting from structural alteration in the Y-X-N loop
Abstract
The Citrobacter freundii isolate CHA, which was responsible for postoperative peritonitis after 10 days of cefepime therapy, displayed a phenotype of resistance consistent with extended-spectrum AmpC (ESAC) β-lactamase. The chromosome-borne bla(AmpC-CHA) gene was amplified and sequenced, revealing five amino acid substitutions, I125V, R148H, Q196H, V305A, and V348A, in the product compared to the sequence of native AmpC. A cloning experiment yielded the Escherichia coli TOP10(pAmpC-CHA) strain, which was resistant to all extended-spectrum cephalosporins (ESCs), including cefepime. To ascertain whether the R148H substitution accounted for the hydrolysis spectrum extension, it was reverted by site-directed mutagenesis. The resulting E. coli TOP10(pAmpC-CHA-H148R) strain was fully susceptible to cefepime, thus confirming that the Arg-148 replacement was mandatory for substrate profile enlargement. To further characterize the phenotypical and biochemical effects induced by the R148H change, it was introduced by site-directed mutagenesis into the CMY-2 β-lactamase, which is structurally related to the chromosome-borne cephalosporinase of C. freundii. The CMY-2-R148H variant conferred increased MICs of ESCs, whereas those of carbapenems were unchanged even in a porin-deficient E. coli strain. Moreover, it exhibited increased catalytic efficiency (k(cat)/K(m)) toward ceftazidime (100-fold) due to an enhanced hydrolysis rate (k(cat)), whereas the enzymatic parameters toward imipenem were unchanged. The structural analysis of the AmpC variant showed that the R148H replacement occurred in the loop containing the Y-X-N motif, which is the counterpart of the SDN loop in class A β-lactamases. This study shows that the Y-X-N loop is a novel hot spot for mutations accounting for hydrolysis spectrum extension in CMY-2-type enzymes.
Figures
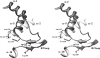
Similar articles
-
Class C β-Lactamases: Molecular Characteristics.Clin Microbiol Rev. 2022 Sep 21;35(3):e0015021. doi: 10.1128/cmr.00150-21. Epub 2022 Apr 18. Clin Microbiol Rev. 2022. PMID: 35435729 Free PMC article. Review.
-
Emergence of a cefepime- and cefpirome-resistant Citrobacter freundii clinical isolate harbouring a novel chromosomally encoded AmpC beta-lactamase, CMY-37.Int J Antimicrob Agents. 2008 Sep;32(3):256-61. doi: 10.1016/j.ijantimicag.2008.04.019. Epub 2008 Jul 10. Int J Antimicrob Agents. 2008. PMID: 18619820
-
In Vivo Evolution of CMY-2 to CMY-33 β-Lactamase in Escherichia coli Sequence Type 131: Characterization of an Acquired Extended-Spectrum AmpC Conferring Resistance to Cefepime.Antimicrob Agents Chemother. 2015 Dec;59(12):7483-8. doi: 10.1128/AAC.01804-15. Epub 2015 Sep 21. Antimicrob Agents Chemother. 2015. PMID: 26392491 Free PMC article.
-
Genetic and biochemical characterization of OXA-1186, a novel OXA-198-type carbapenemase hydrolysing cephalosporins in Citrobacter freundii.J Antimicrob Chemother. 2024 Dec 2;79(12):3186-3190. doi: 10.1093/jac/dkae339. J Antimicrob Chemother. 2024. PMID: 39319679
-
Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens.Int J Med Microbiol. 2010 Aug;300(6):371-9. doi: 10.1016/j.ijmm.2010.04.005. Epub 2010 May 27. Int J Med Microbiol. 2010. PMID: 20537585 Review.
Cited by
-
N152G, -S, and -T substitutions in CMY-2 β-lactamase increase catalytic efficiency for cefoxitin and inactivation rates for tazobactam.Antimicrob Agents Chemother. 2013 Apr;57(4):1596-602. doi: 10.1128/AAC.01334-12. Epub 2013 Jan 14. Antimicrob Agents Chemother. 2013. PMID: 23318801 Free PMC article.
-
Interactions of oximino-substituted boronic acids and β-lactams with the CMY-2-derived extended-spectrum cephalosporinases CMY-30 and CMY-42.Antimicrob Agents Chemother. 2013 Feb;57(2):968-76. doi: 10.1128/AAC.01620-12. Epub 2012 Dec 10. Antimicrob Agents Chemother. 2013. PMID: 23229484 Free PMC article.
-
Class C β-Lactamases: Molecular Characteristics.Clin Microbiol Rev. 2022 Sep 21;35(3):e0015021. doi: 10.1128/cmr.00150-21. Epub 2022 Apr 18. Clin Microbiol Rev. 2022. PMID: 35435729 Free PMC article. Review.
-
Impact of extended-spectrum chromosomal AmpC (ESAC) of Escherichia coli on susceptibility to cefiderocol.Microbiol Spectr. 2024 Jul 2;12(7):e0070424. doi: 10.1128/spectrum.00704-24. Epub 2024 Jun 11. Microbiol Spectr. 2024. PMID: 38860818 Free PMC article.
-
In vivo emergence of resistance to ceftazidime/avibactam through modification of chromosomal AmpC β-lactamase in Klebsiella aerogenes.Antimicrob Agents Chemother. 2024 Dec 5;68(12):e0130724. doi: 10.1128/aac.01307-24. Epub 2024 Nov 6. Antimicrob Agents Chemother. 2024. PMID: 39503481 Free PMC article.
References
-
- Ahmed AM, Shimamoto T. 2008. Emergence of a cefepime- and cefpirome-resistant Citrobacter freundii clinical isolate harbouring a novel chromosomally encoded AmpC β-lactamase, CMY-37. Int. J. Antimicrob. Agents 32:256–261 - PubMed
-
- Barnaud G, et al. 2001. Extension of resistance to cefepime and cefpirome associated to a six amino acid deletion in the H-10 helix of the cephalosporinase of an Enterobacter cloacae clinical isolate. FEMS Microbiol. Lett. 195:185–190 - PubMed
-
- Bauernfeind A, Chong Y, Schweighart S. 1989. Extended broad spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection 17:316–321 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

