Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study
- PMID: 22232285
- PMCID: PMC3294909
- DOI: 10.1128/AAC.05658-11
Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study
Abstract
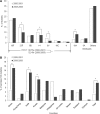
Antimicrobial resistance in Streptococcus pneumoniae remains a serious concern worldwide, particularly in Asian countries, despite the introduction of heptavalent pneumococcal conjugate vaccine (PCV7). The Asian Network for Surveillance of Resistant Pathogens (ANSORP) performed a prospective surveillance study of 2,184 S. pneumoniae isolates collected from patients with pneumococcal infections from 60 hospitals in 11 Asian countries from 2008 to 2009. Among nonmeningeal isolates, the prevalence rate of penicillin-nonsusceptible pneumococci (MIC, ≥ 4 μg/ml) was 4.6% and penicillin resistance (MIC, ≥ 8 μg/ml) was extremely rare (0.7%). Resistance to erythromycin was very prevalent in the region (72.7%); the highest rates were in China (96.4%), Taiwan (84.9%), and Vietnam (80.7%). Multidrug resistance (MDR) was observed in 59.3% of isolates from Asian countries. Major serotypes were 19F (23.5%), 23F (10.0%), 19A (8.2%), 14 (7.3%), and 6B (7.3%). Overall, 52.5% of isolates showed PCV7 serotypes, ranging from 16.1% in Philippines to 75.1% in Vietnam. Serotypes 19A (8.2%), 3 (6.2%), and 6A (4.2%) were the most prominent non-PCV7 serotypes in the Asian region. Among isolates with serotype 19A, 86.0% and 79.8% showed erythromycin resistance and MDR, respectively. The most remarkable findings about the epidemiology of S. pneumoniae in Asian countries after the introduction of PCV7 were the high prevalence of macrolide resistance and MDR and distinctive increases in serotype 19A.
Figures
References
-
- Albrich WC, Baughman W, Schmotzer B, Farley MM. 2007. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin. Infect. Dis. 44:1569–1576 - PubMed
-
- Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. M07-A8 Clinical and Laboratory Standards Institute, Wayne, PA
-
- Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. M100-S21, vol. 31 Clinical and Laboratory Standards Institute, Wayne, PA
-
- Gertz RE, Jr, et al. 2010. Increased penicillin nonsusceptibility of nonvaccine-serotype invasive pneumococci other than serotypes 19A and 6A in post-7-valent conjugate vaccine era. J. Infect. Dis. 201:770–775 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

