Dependence of corneal stem/progenitor cells on ocular surface innervation
- PMID: 22232434
- PMCID: PMC3317425
- DOI: 10.1167/iovs.11-8438
Dependence of corneal stem/progenitor cells on ocular surface innervation
Abstract
Purpose: Neurotrophic keratopathy (NK) is a corneal degeneration associated with corneal nerve dysfunction. It can cause corneal epithelial defects, stromal thinning, and perforation. However, it is not clear if and to which extent epithelial stem cells are affected in NK. The purpose of this study was to identify the relationship between corneolimbal epithelial progenitor/stem cells and sensory nerves using a denervated mouse model of NK.
Methods: NK was induced in mice by electrocoagulation of the ophthalmic branch of the trigeminal nerve. The absence of corneal nerves was confirmed with β-III tubulin immunostaining and blink reflex test after 7 days. ATP-binding cassette subfamily G member 2 (ABCG2), p63, and hairy enhancer of split 1 (Hes1) were chosen as corneolimbal stem/progenitor cell markers and assessed in denervated mice versus controls by immunofluorescent microscopy and real-time PCR. In addition, corneolimbal stem/progenitor cells were detected as side population cells using flow cytometry, and colony-forming efficiency assay was performed to assess their function.
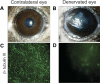
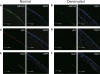
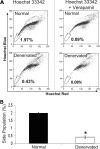
Results: ABCG2, p63, and Hes1 immunostaining were significantly decreased in denervated eyes after 7 days. Similarly, the expression levels of ABCG2, p63, K15, Hes1, and N-cadherin transcripts were also significantly decreased in denervated eyes. Stem/progenitor cells measured as side population from NK mice were decreased by approximately 75% compared with normals. In addition, the authors found a significant (P = 0.038) reduction in colony-forming efficiency of stem/progenitor cells harvested from denervated eyes.
Conclusions: Corneolimbal stem/progenitor cells are significantly reduced after depletion of sensory nerves. The data suggest a critical role of innervation in maintaining stem cells and/or the stem cell niche.
Figures


Similar articles
-
A novel mouse model for neurotrophic keratopathy: trigeminal nerve stereotactic electrolysis through the brain.Invest Ophthalmol Vis Sci. 2011 Apr 19;52(5):2532-9. doi: 10.1167/iovs.10-5688. Print 2011 Apr. Invest Ophthalmol Vis Sci. 2011. PMID: 21071731 Free PMC article.
-
A comparison of stem cell-related gene expression in the progenitor-rich limbal epithelium and the differentiating central corneal epithelium.Mol Vis. 2011;17:2102-17. Epub 2011 Aug 10. Mol Vis. 2011. PMID: 21850186 Free PMC article.
-
Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells.Exp Eye Res. 2008 Jan;86(1):34-40. doi: 10.1016/j.exer.2007.09.003. Epub 2007 Sep 15. Exp Eye Res. 2008. PMID: 17980361 Free PMC article.
-
Ocular surface epithelial and stem cell development.Int J Dev Biol. 2004;48(8-9):981-91. doi: 10.1387/ijdb.041876jw. Int J Dev Biol. 2004. PMID: 15558489 Review.
-
Identification and characterization of limbal stem cells.Exp Eye Res. 2005 Sep;81(3):247-64. doi: 10.1016/j.exer.2005.02.016. Exp Eye Res. 2005. PMID: 16051216 Review.
Cited by
-
Neural Hedgehog signaling maintains stem cell renewal in the sensory touch dome epithelium.Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):7195-200. doi: 10.1073/pnas.1504177112. Epub 2015 May 26. Proc Natl Acad Sci U S A. 2015. PMID: 26015562 Free PMC article.
-
Expansion of Human Limbal Epithelial Stem/Progenitor Cells Using Different Human Sera: A Multivariate Statistical Analysis.Int J Mol Sci. 2020 Aug 25;21(17):6132. doi: 10.3390/ijms21176132. Int J Mol Sci. 2020. PMID: 32854428 Free PMC article.
-
Expression of pluripotency factors in larval epithelia of the frog Xenopus: evidence for the presence of cornea epithelial stem cells.Dev Biol. 2013 Feb 15;374(2):281-94. doi: 10.1016/j.ydbio.2012.12.005. Epub 2012 Dec 26. Dev Biol. 2013. PMID: 23274420 Free PMC article.
-
Neprilysin inhibition promotes corneal wound healing.Sci Rep. 2018 Sep 26;8(1):14385. doi: 10.1038/s41598-018-32773-9. Sci Rep. 2018. PMID: 30258206 Free PMC article.
-
Corneal myofibroblasts inhibit regenerating nerves during wound healing.Sci Rep. 2018 Aug 28;8(1):12945. doi: 10.1038/s41598-018-30964-y. Sci Rep. 2018. PMID: 30154512 Free PMC article.
References
-
- Kinoshita S, Friend J, Thoft RA. Sex chromatin of donor corneal epithelium in rabbits. Invest Ophthalmol Vis Sci. 1981;21:434–441 - PubMed
-
- Buck RC. Measurement of centripetal migration of normal corneal epithelial cells in the mouse. Invest Ophthalmol Vis Sci. 1985;26:1296–1299 - PubMed
-
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209 - PubMed
-
- Kruse FE. Stem cells and corneal epithelial regeneration. Eye. 1994;8:170–183 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

