Proteome analyses of hydrogen-producing hyperthermophilic archaeon Thermococcus onnurineus NA1 in different one-carbon substrate culture conditions
- PMID: 22232491
- PMCID: PMC3433910
- DOI: 10.1074/mcp.M111.015420
Proteome analyses of hydrogen-producing hyperthermophilic archaeon Thermococcus onnurineus NA1 in different one-carbon substrate culture conditions
Abstract
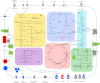
Thermococcus onnurineus NA1, a sulfur-reducing hyperthermophilic archaeon, is capable of H(2)-producing growth, considered to be hydrogenogenic carboxydotrophy. Utilization of formate as a sole energy source has been well studied in T. onnurineus NA1. However, whether formate can be used as its carbon source remains unknown. To obtain a global view of the metabolic characteristics of H(2)-producing growth, a quantitative proteome analysis of T. onnurineus NA1 grown on formate, CO, and starch was performed by combining one-dimensional SDS-PAGE with nano UPLC-MS(E). A total of 587 proteins corresponding to 29.7% of the encoding genes were identified, and the major metabolic pathways (especially energy metabolism) were characterized at the protein level. Expression of glycolytic enzymes was common but more highly induced in starch-grown cells. In contrast, enzymes involved in key steps of the gluconeogenesis and pentose phosphate pathways were strongly up-regulated in formate-grown cells, suggesting that formate could be utilized as a carbon source by T. onnurineus NA1. In accordance with the genomic analysis, comprehensive proteomic analysis also revealed a number of hydrogenase clusters apparently associated with formate metabolism. On the other hand, CODH and CO-induced hydrogenases belonging to the Hyg4-II cluster, as well as sulfhydrogenase-I and Mbx, were prominently expressed during CO culture. Our data suggest that CO can be utilized as a sole energy source for H(2) production via an electron transport mechanism and that CO(2) produced from catabolism or CO oxidation by CODH and CO-induced hydrogenases may subsequently be assimilated into the organic carbon. Overall, proteomic comparison of formate- and CO-grown cells with starch-grown cells revealed that a single carbon compound, such as formate and CO, can be utilized as an efficient substrate to provide cellular carbon and/or energy by T. onnurineus NA1.
Figures

References
-
- Holden J. F., Takai K., Summit M., Bolton S., Zyskowski J., Baross J. A. (2001) Diversity among three novel groups of hyperthermophilic deep-sea Thermococcus species from three sites in the northeastern pacific ocean. FEMS Microbiol. Ecol. 36, 51–60 - PubMed
-
- Xue Y., Xu Y., Liu Y., Ma Y., Zhou P. (2001) Thermoanaerobacter tengcongensis sp. nov., a novel anaerobic, saccharolytic, thermophilic bacterium isolated from a hot spring in Tengcong, China. Int. J. Syst. Evol. Microbiol. 51, 1335–1341 - PubMed
-
- Miroshnichenko M. L., Hippe H., Stackebrandt E., Kostrikina N. A., Chernyh N. A., Jeanthon C., Nazina T. N., Belyaev S. S., Bonch-Osmolovskaya E. A. (2001) Isolation and characterization of Thermococcus sibiricus sp. nov. from a Western Siberia high-temperature oil reservoir. Extremophiles 5, 85–91 - PubMed
-
- Bertoldo C., Antranikian G. (2006) The order Thermococcales, in The Prokaryotes (Dworkin M., Falkow S., Rosenberg E., Schleifer K. H., Stackebrandt E., eds) Vol. 3, 3rd Ed., pp. 69–81, Springer, New York
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

