cAMP-specific phosphodiesterases 8A and 8B, essential regulators of Leydig cell steroidogenesis
- PMID: 22232524
- PMCID: PMC3310417
- DOI: 10.1124/mol.111.076125
cAMP-specific phosphodiesterases 8A and 8B, essential regulators of Leydig cell steroidogenesis
Abstract
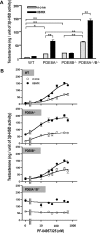
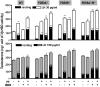
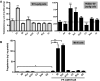
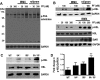
Phosphodiesterase (PDE) 8A and PDE8B are high-affinity, cAMP-specific phosphodiesterases that are highly expressed in Leydig cells. PDE8A is largely associated with mitochondria, whereas PDE8B is broadly distributed in the cytosol. We used a new, PDE8-selective inhibitor, PF-04957325, and genetically ablated PDE8A(-/-), PDE8B(-/-) and PDE8A(-/-)/B(-/-) mice to determine roles for these PDEs in the regulation of testosterone production. PF-04957325 treatment of WT Leydig cells or MA10 cells increased steroid production but had no effect in PDE8A (-/-)/B(-/-) double-knockout cells, confirming the selectivity of the drug. Moreover, under basal conditions, cotreatment with PF-04957325 plus rolipram, a PDE4-selective inhibitor, synergistically potentiated steroid production. These results suggest that the pool(s) of cAMP regulating androgen production are controlled by PDE8s working in conjunction with PDE4. Likewise, PDE8A (-/-)/B(-/-) cells had higher testosterone production than cells from either PDE8A(-/-) or PDE8B(-/-) mice, suggesting that both PDE8s work in concert to regulate steroid production. We further demonstrate that combined inhibition of PDE8s and PDE4 greatly increased PKA activity including phosphorylation of cholesterol-ester hydrolase (CEH)/hormone-sensitive lipase (HSL). CEH/HSL phosphorylation also was increased in PDE8A(-/-)/B(-/-) cells compared with WT cells. Finally, combined inhibition of PDE8s and PDE4 increased the expression of steroidogenic acute regulatory (StAR) protein. Together these findings suggest that both PDE8A and PDE8B play essential roles to maintain low cAMP levels, thereby suppressing resting steroidogenesis by keeping CEH/HSL inactive and StAR protein expression low. They also suggest that in order for PDE inhibitor therapy to be an effective stimulator of steroidogenesis, both PDE8 isozymes and PDE4 need to be simultaneously targeted.
Figures



References
-
- Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C. (1998) Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273:215–221 - PubMed
-
- Arakane F, King SR, Du Y, Kallen CB, Walsh LP, Watari H, Stocco DM, Strauss JF., 3rd (1997) Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem 272:32656–32662 - PubMed
-
- Ascoli M. (1981) Characterization of several clonal lines of cultured Leydig tumor cells: gonadotropin receptors and steroidogenic responses. Endocrinology 108:88–95 - PubMed
-
- Carlucci A, Lignitto L, Feliciello A. (2008) Control of mitochondria dynamics and oxidative metabolism by cAMP, AKAPs and the proteasome. Trends Cell Biol 18:604–613 - PubMed
-
- Conti M, Beavo J. (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

