Tyrosine-phosphorylated galectin-3 protein is resistant to prostate-specific antigen (PSA) cleavage
- PMID: 22232548
- PMCID: PMC3285300
- DOI: 10.1074/jbc.C111.331686
Tyrosine-phosphorylated galectin-3 protein is resistant to prostate-specific antigen (PSA) cleavage
Abstract
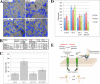
Galectin-3 is a chimeric carbohydrate-binding protein, which interacts with cell surface carbohydrate-containing molecules and extracellular matrix glycoproteins and has been implicated in various biological processes such as cell growth, angiogenesis, motility, and metastasis. It is expressed in a wide range of tumor cells and is associated with tumor progression. The functions of galectin-3 are dependent on its localization and post-translational modifications such as cleavage and phosphorylation. Recently, we showed that galectin-3 Tyr-107 is phosphorylated by c-Abl; concomitantly, it was also shown that galectin-3 can be cleaved at this site by prostate-specific antigen (PSA), a chymotrypsin-like serine protease, after Tyr-107, resulting in loss of galectin-3 multivalency while preserving its carbohydrate binding activity. Galectin-3 is largely a monomer in solution but may form a homodimer by self-association through its carbohydrate recognition domain, whereas, in the presence of a ligand, galectin-3 polymerizes up to pentamers utilizing its N-terminal domain. Oligomerization is a unique feature of secreted galectin-3, which allows its function by forming ordered galectin-glycan structures, i.e. lattices, on the cell surface or through direct engagement of specific cell surface glycoconjugates by traditional ligand-receptor binding. We questioned whether Tyr-107 phosphorylation by c-Abl affects galectin-3 cleavage by PSA. The data suggest a role for galectin-3 in prostate cells associated with increased activity of c-Abl kinase and loss of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) activity. In addition, the ratio of phosphorylated/dephosphorylated galectin-3 might be used as a complementary value to that of PSA for prognosis of prostate cancer and a novel therapeutic target for the treatment of prostate cancer.
Figures

References
-
- Huflejt M. E., Turck C. W., Lindstedt R., Barondes S. H., Leffler H. (1993) L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J. Biol. Chem. 268, 26712–26718 - PubMed
-
- Barondes S. H., Cooper D. N., Gitt M. A., Leffler H. (1994) Galectins: structure and function of a large family of animal lectins. J. Biol. Chem. 269, 20807–20810 - PubMed
-
- Ahmad N., Gabius H. J., André S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C. F. (2004) Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279, 10841–10847 - PubMed
-
- Barondes S. H., Castronovo V., Cooper D. N., Cummings R. D., Drickamer K., Feizi T., Gitt M. A., Hirabayashi J., Hughes C., Kasai K. (1994) Galectins: a family of animal β-galactoside-binding lectins. Cell 76, 597–598 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

