Flotillin-1/reggie-2 protein plays dual role in activation of receptor-tyrosine kinase/mitogen-activated protein kinase signaling
- PMID: 22232557
- PMCID: PMC3293549
- DOI: 10.1074/jbc.M111.287599
Flotillin-1/reggie-2 protein plays dual role in activation of receptor-tyrosine kinase/mitogen-activated protein kinase signaling
Abstract
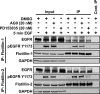
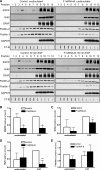
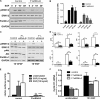
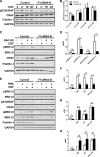
Our previous work has shown that the membrane microdomain-associated flotillin proteins are potentially involved in epidermal growth factor (EGF) receptor signaling. Here we show that knockdown of flotillin-1/reggie-2 results in reduced EGF-induced phosphorylation of specific tyrosines in the EGF receptor (EGFR) and in inefficient activation of the downstream mitogen-activated protein (MAP) kinase and Akt signaling. Although flotillin-1 has been implicated in endocytosis, its depletion affects neither the endocytosis nor the ubiquitination of the EGFR. However, EGF-induced clustering of EGFR at the cell surface is altered in cells lacking flotillin-1. Furthermore, we show that flotillins form molecular complexes with EGFR in an EGF/EGFR kinase-independent manner. However, knockdown of flotillin-1 appears to affect the activation of the downstream MAP kinase signaling more directly. We here show that flotillin-1 forms a complex with CRAF, MEK1, ERK, and KSR1 (kinase suppressor of RAS) and that flotillin-1 knockdown leads to a direct inactivation of ERK1/2. Thus, flotillin-1 plays a direct role during both the early phase (activation of the receptor) and late (activation of MAP kinases) phase of growth factor signaling. Our results here unveil a novel role for flotillin-1 as a scaffolding factor in the regulation of classical MAP kinase signaling. Furthermore, our results imply that other receptor-tyrosine kinases may also rely on flotillin-1 upon activation, thus suggesting a general role for flotillin-1 as a novel factor in receptor-tyrosine kinase/MAP kinase signaling.
Figures




Similar articles
-
Increased activity of mitogen activated protein kinase pathway in flotillin-2 knockout mouse model.Cell Signal. 2014 Feb;26(2):198-207. doi: 10.1016/j.cellsig.2013.11.001. Epub 2013 Nov 9. Cell Signal. 2014. PMID: 24216609
-
Phosphatidylinositol 3-Kinase dependent upregulation of the epidermal growth factor receptor upon Flotillin-1 depletion in breast cancer cells.BMC Cancer. 2013 Dec 5;13:575. doi: 10.1186/1471-2407-13-575. BMC Cancer. 2013. PMID: 24304721 Free PMC article.
-
Endocytosis separates EGF receptors from endogenous fluorescently labeled HRas and diminishes receptor signaling to MAP kinases in endosomes.Proc Natl Acad Sci U S A. 2016 Feb 23;113(8):2122-7. doi: 10.1073/pnas.1520301113. Epub 2016 Feb 8. Proc Natl Acad Sci U S A. 2016. PMID: 26858456 Free PMC article.
-
Scaffolding microdomains and beyond: the function of reggie/flotillin proteins.Cell Mol Life Sci. 2005 Oct;62(19-20):2228-40. doi: 10.1007/s00018-005-5166-4. Cell Mol Life Sci. 2005. PMID: 16091845 Free PMC article. Review.
-
Flotillins in membrane trafficking and physiopathology.Biol Cell. 2025 Jan;117(1):e2400134. doi: 10.1111/boc.202400134. Biol Cell. 2025. PMID: 39877933 Free PMC article. Review.
Cited by
-
Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies.Signal Transduct Target Ther. 2023 Dec 18;8(1):455. doi: 10.1038/s41392-023-01705-z. Signal Transduct Target Ther. 2023. PMID: 38105263 Free PMC article. Review.
-
Flotillins Regulate Focal Adhesions by Interacting with α-Actinin and by Influencing the Activation of Focal Adhesion Kinase.Cells. 2018 Apr 7;7(4):28. doi: 10.3390/cells7040028. Cells. 2018. PMID: 29642469 Free PMC article.
-
Regulation of cargo transfer between ESCRT-0 and ESCRT-I complexes by flotillin-1 during endosomal sorting of ubiquitinated cargo.Oncogenesis. 2017 Jun 5;6(6):e344. doi: 10.1038/oncsis.2017.47. Oncogenesis. 2017. PMID: 28581508 Free PMC article.
-
Transcriptional regulation of flotillins by the extracellularly regulated kinases and retinoid X receptor complexes.PLoS One. 2012;7(9):e45514. doi: 10.1371/journal.pone.0045514. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23029064 Free PMC article.
-
FLOT1 knockdown inhibits growth of AML cells through triggering apoptosis and pyroptosis.Ann Hematol. 2023 Mar;102(3):583-595. doi: 10.1007/s00277-023-05103-x. Epub 2023 Jan 26. Ann Hematol. 2023. PMID: 36697954
References
-
- Carpentier J. L., Gorden P., Anderson R. G., Goldstein J. L., Brown M. S., Cohen S., Orci L. (1982) Co-localization of 125I-epidermal growth factor and ferritin low density lipoprotein in coated pits. A quantitative electron microscopic study in normal and mutant human fibroblasts. J. Cell Biol. 95, 73–77 - PMC - PubMed
-
- Hanover J. A., Willingham M. C., Pastan I. (1984) Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell 39, 283–293 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

