ERK pathway activation bidirectionally affects visual recognition memory and synaptic plasticity in the perirhinal cortex
- PMID: 22232579
- PMCID: PMC3246765
- DOI: 10.3389/fnbeh.2011.00084
ERK pathway activation bidirectionally affects visual recognition memory and synaptic plasticity in the perirhinal cortex
Abstract
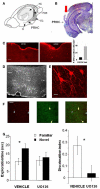
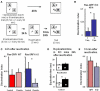
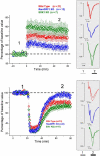
ERK 1,2 pathway mediates experience-dependent gene transcription in neurons and several studies have identified its pivotal role in experience-dependent synaptic plasticity and in forms of long term memory involving hippocampus, amygdala, or striatum. The perirhinal cortex (PRHC) plays an essential role in familiarity-based object recognition memory. It is still unknown whether ERK activation in PRHC is necessary for recognition memory consolidation. Most important, it is unknown whether by modulating the gain of the ERK pathway it is possible to bidirectionally affect visual recognition memory and PRHC synaptic plasticity. We have first pharmacologically blocked ERK activation in the PRHC of adult mice and found that this was sufficient to impair long term recognition memory in a familiarity-based task, the object recognition task (ORT). We have then tested performance in the ORT in Ras-GRF1 knock-out (KO) mice, which exhibit a reduced activation of ERK by neuronal activity, and in ERK1 KO mice, which have an increased activation of ERK2 and exhibit enhanced striatal plasticity and striatal mediated memory. We found that Ras-GRF1 KO mice have normal short term memory but display a long term memory deficit; memory reconsolidation is also impaired. On the contrary, ERK1 KO mice exhibit a better performance than WT mice at 72 h retention interval, suggesting a longer lasting recognition memory. In parallel with behavioral data, LTD was strongly reduced and LTP was significantly smaller in PRHC slices from Ras-GRF1 KO than in WT mice while enhanced LTP and LTD were found in PRHC slices from ERK1 KO mice.
Keywords: ERK1,2; perirhinal cortex; recognition memory; synaptic plasticity.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

